Session Information
Date: Tuesday, October 23, 2018
Title: Epidemiology and Public Health Poster III: SLE, SSc, APS, PsA, and Other Rheumatic Diseases
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Methotrexate (MTX) is commonly used to treat psoriatic arthritis (PsA), yet data supporting its effectiveness in PsA are limited. This analysis describes real-world dosing, treatment patterns, and administration modifications in PsA patients initiating MTX.
Methods: Adults with PsA were selected if they initiated MTX (index date) between January 1, 2012, and September 30, 2016, had ≥12 months pre- and post-index continuous enrollment in the IQVIA™ PharMetrics Plus real-world data adjudicated claims database, and had no history of systemic disease-modifying PsA therapies 12 months pre-index. Concomitant drug use with known MTX interactions was captured along with rates of MTX discontinuation, switch, initiation of combination therapy, and changes to dose and/or administration. Concomitant drug use was defined as ≥7 days’ supply overlap. Discontinuation was defined as a ≥60-day lapse in therapy. Switching was defined as the presence of a new therapy, with a <60-day MTX overlap and MTX discontinuation. Combination therapy was defined as the addition of a PsA agent to MTX, with a ≥60-day overlap. Patients were followed for 12 months after their index date.
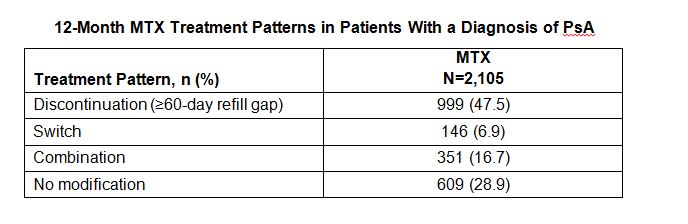
Results: A total of 2,105 MTX patients were included. Mean (SD) age was 48.0 (10.3) years and 51% were male. Mean follow-up was 30.1 months. During the pre-index period, 48.2%, 54.9%, and 51.5% used topical steroids, systemic steroids, and non-steroidal anti-inflammatory drugs, respectively. Rates of comorbidities that could increase patients’ risk of MTX toxicity were hyperlipidemia (33.5%), diabetes (13.3%), and obesity (13.9%). Other comorbidities included osteoarthritis (54.7%), hypertension (33.2%), and fibromyalgia (17.9%). More than half of MTX patients (56.5%) had a concomitant prescription with known MTX interaction. Mean (SD) duration of MTX monotherapy was 186.0 days (129.7). During follow-up, 57.6% of patients had ≥1 increase in dose and 31.6% had ≥1 decrease in dose, while 108 (5.1%) patients changed from oral to subcutaneous injection. Discontinuation was the most frequent treatment modification, followed by combination therapy (Table). Mean (SD) time to discontinuation, switch, and combination therapy was 99.8 days (75.5), 112.2 (60.0) days, and 150.8 (84.7) days, respectively.
Conclusion: During follow-up, half of the MTX users discontinued therapy, had concomitant therapy with known MTX interactions, and had at least 1 increase in MTX dose. More research is needed to better understand the impact of treatment patterns on healthcare outcomes among PsA patients initiating treatment with MTX.
To cite this abstract in AMA style:
Ung B, Hines D, Mehta S, Pelletier C, Wang X, Wade R. Real-World Evaluation of Treatment Patterns of Methotrexate Users in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/real-world-evaluation-of-treatment-patterns-of-methotrexate-users-in-psoriatic-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-evaluation-of-treatment-patterns-of-methotrexate-users-in-psoriatic-arthritis/