Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
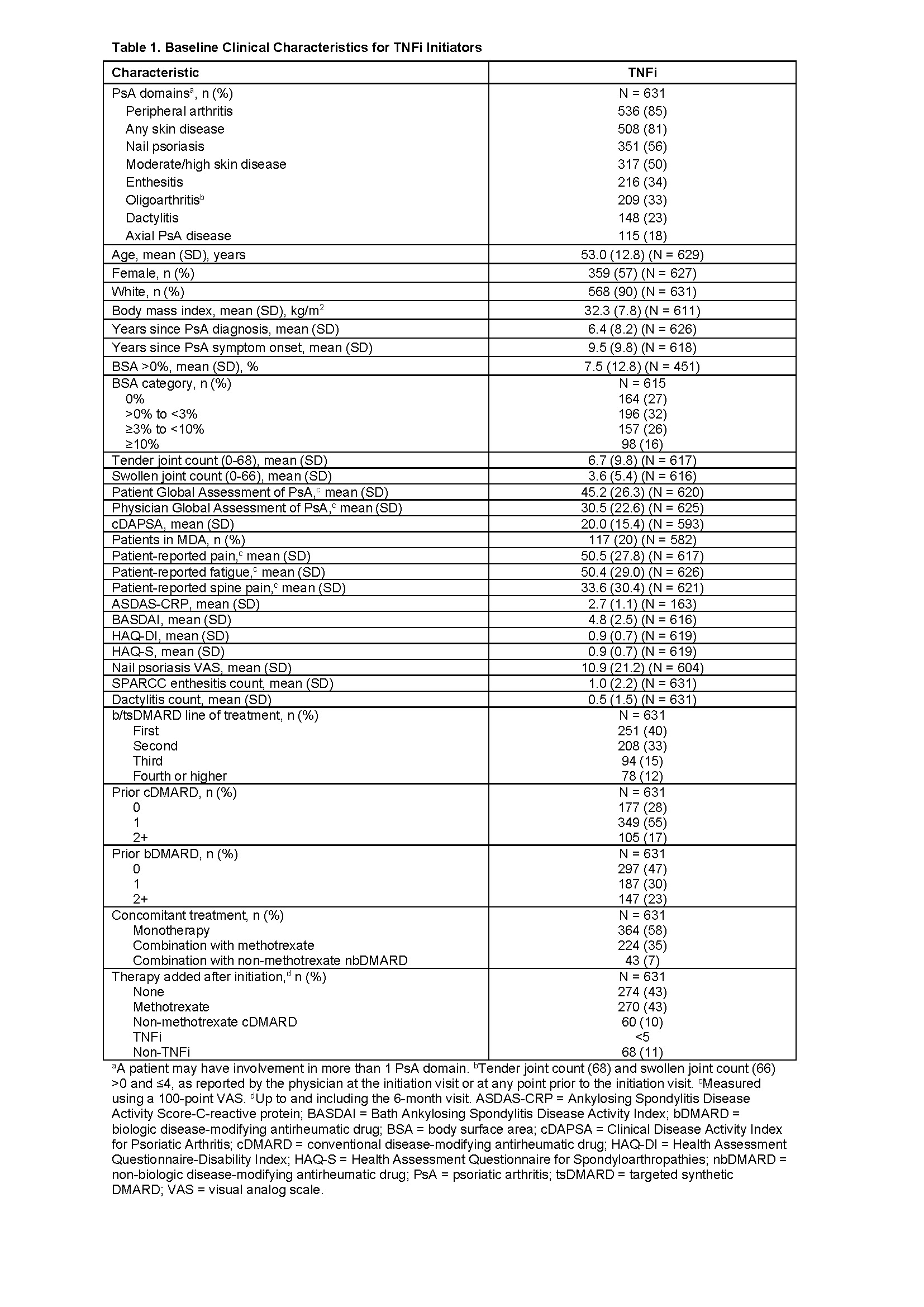
Background/Purpose: The burden of PsA and its chronic symptoms may have considerable impact on patient function and quality of life. Real-world research is limited on treatment response in PsA patients with different manifestations of PsA who are treated with a biologic DMARD. The objective of this study was to describe patient characteristics and disease outcomes within different PsA domains for patients initiating treatment with a TNF inhibitor (TNFi).
Methods: The CorEvitas PsA/Spondyloarthritis (SpA) Registry is a prospective, observational registry for patients with PsA or SpA under care of a rheumatologist. This study included adults with PsA who initiated treatment with a TNFi (adalimumab, etanercept, certolizumab pegol, infliximab, golimumab) from January 2013‒January 2020. We present unadjusted change in disease activity and patient-reported measures from baseline to 6 months for each PsA domain (enthesitis, dactylitis, peripheral arthritis, oligoarthritis, axial PsA disease, nail psoriasis, any skin disease, and moderate/high skin disease). We examined median time to therapy discontinuation and proportions of patients persistent on TNFi at 6, 12, and 24 months.
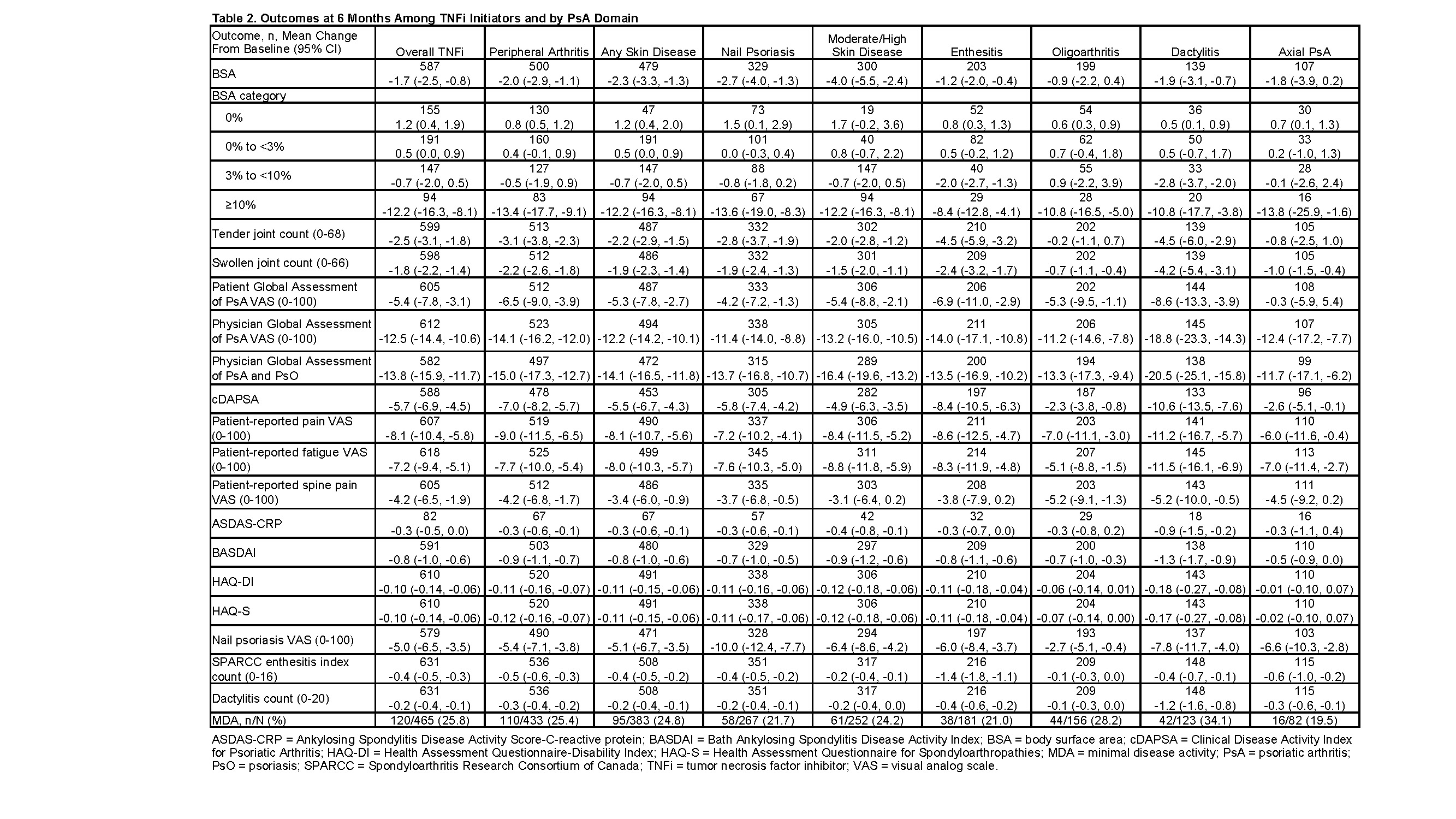
Results: A total of 631 patients with PsA initiated TNFi treatment. Peripheral arthritis (85%), skin disease (81%), and nail psoriasis (56%) were the most common PsA manifestations (Table 1). Most patients were receiving first- or second-line therapy (73%) (Table 1). At 6 months, improvements were observed for the overall population of TNFi initiators and within the subgroups defined by PsA domains (Table 2). Mean change from baseline in scores for Physician Global Assessment of Disease Activity (100-point VAS) ranged from −11.2 in patients with oligoarthritis to −18.8 in patients with dactylitis (Table 2). Similar patterns of improvement were seen in a subanalysis of patients treated with etanercept.
Among the overall population of patients who were not in minimal disease activity (MDA) at baseline, 25.8% achieved MDA at 6 months. Among the subtypes, the proportion of patients achieving MDA ranged from 19.5% in patients with axial PsA to 34.1% in patients with dactylitis (Table 2). Persistency at 6, 12, and 24 months for patients treated with a TNFi was 0.75, 0.57, and 0.39, respectively, and was similar across subgroups of patients with PsA manifestations (Table 3). Time to discontinuation was shortest in patients with enthesitis and axial PsA (12 and 13 months, respectively) and longest in those with oligoarthritis (20 months) (Table 3).
Conclusion: This analysis highlights the heterogeneity in presentation of PsA domains. In this real-world population, improvement was seen across all PsA domains after initiation of TNFi treatment.
To cite this abstract in AMA style:
Mease P, Blachley T, O’Brien J, Middaugh N, Kricorian G, Stryker S, Collier D, Ogdie-Beatty A. Real-World Efficacy of TNF Inhibitors for Treatment of Psoriatic Arthritis by Disease Domains: 6-Month Findings from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/real-world-efficacy-of-tnf-inhibitors-for-treatment-of-psoriatic-arthritis-by-disease-domains-6-month-findings-from-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-efficacy-of-tnf-inhibitors-for-treatment-of-psoriatic-arthritis-by-disease-domains-6-month-findings-from-the-corevitas-psoriatic-arthritis-spondyloarthritis-registry/