Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Psoriatic arthritis (PsA) is a heterogenous inflammatory disease that can cause substantial impairment in quality of life in affected patients. The Group for Research and Assessment of Psoriasis and PsA (GRAPPA) domain-based treatment recommendations for PsA highlight 6 key domains: peripheral arthritis, axial disease, enthesitis, dactylitis, skin psoriasis, and nail disease. Upadacitinib (UPA) has demonstrated efficacy versus placebo across key domains in clinical trials in patients with PsA, but real-world data are limited. In this study, we evaluated changes in disease activity measures and patient-reported outcomes (PROs) in patients with PsA who initiated UPA and persisted on therapy for 6 and 12 months overall in a real-world setting.
Methods: Patients with PsA in the CorEvitas PsA/Spondyloarthritis Registry who initiated UPA during or after December 2021 and had a baseline visit associated with UPA initiation were included. Disease activity and PROs were summarized at baseline; response rates (n [%]; 95% [confidence intervals] CI) for patients who were not in the outcomes state at baseline were calculated at 6 and 12 months. Outcomes included achievement of tender joint count ≤ 1, swollen joint count ≤ 1, patient spine pain (0–100 visual analog scale [VAS]) ≥ 10-point decrease, enthesitis count = 0, dactylitis count = 0, < 3% body surface area (BSA) affected by psoriasis, and nail psoriasis (0–100 VAS) = 0. Results are reported for all initiators, for those persisting on UPA treatment at 6 or 12 months, and for a subset of patients with prior TNFi-experience.
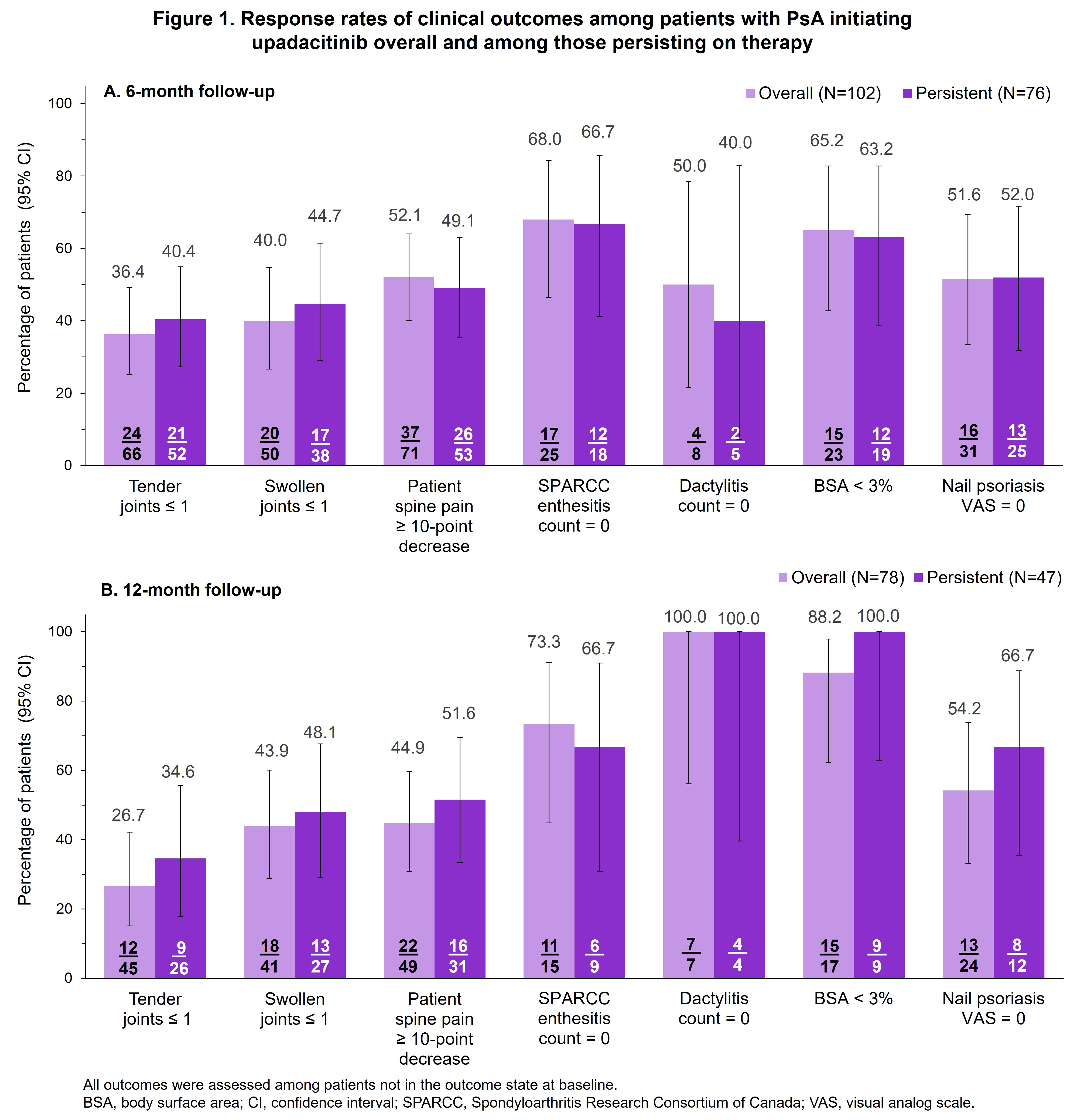
Results: This analysis included 214 patients who initiated UPA. Of these 48% (n/N, 102/214) and 36% (78/214) had 6-month and 12-month follow-up visit data available, respectively. Among all initiators, 65% (140/214) were female, mean age was 54 years, 41% (88/214) were conventional synthetic DMARD-naïve and 61% (130/214) had previously used ≥2 biologic or targeted synthetic DMARDs. Among those with respective follow-up visits, 75% (76/102) and 60% (47/78) were persistent on therapy at 6 and 12 months. At the 6-month follow-up visit, 36% (24/66) of all initiators achieved ≤ 1 tender joint, 40% (20/50) achieved ≤ 1 swollen joint, 52% (37/71) experienced a ≥10-point decrease in spine pain, 68% (17/25) achieved resolution of enthesitis, 50% (4/8) achieved resolution of dactylitis, 65% (15/23) achieved BSA < 3%, and 52% (16/31) achieved nail psoriasis VAS = 0 (Figure 1). Similar trends were seen in the subset of patients who were TNF-experienced (Figure 2), and in those who persisted on UPA therapy for 6 and 12 months.
Conclusion: This real-world study of patients with PsA showed that those who initiated UPA had improvements in disease activity and PROs consistent with key GRAPPA domains at 6 and 12 months. Similar results were observed in the subset of TNFi-experienced patients.
To cite this abstract in AMA style:
Mease P, Ye X, Saffore C, Iyile T, Middaugh N, Blachley T, Eliot M, Ogdie A. Real-world Effectiveness of Upadacitinib on Group for Research and Assessment of Psoriasis and Psoriatic Arthritis Core Domains for Patients with Psoriatic Arthritis: Data from the CorEvitas Psoriatic Arthritis/Spondyloarthritis Registry [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/real-world-effectiveness-of-upadacitinib-on-group-for-research-and-assessment-of-psoriasis-and-psoriatic-arthritis-core-domains-for-patients-with-psoriatic-arthritis-data-from-the-corevitas-psoriatic/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/real-world-effectiveness-of-upadacitinib-on-group-for-research-and-assessment-of-psoriasis-and-psoriatic-arthritis-core-domains-for-patients-with-psoriatic-arthritis-data-from-the-corevitas-psoriatic/

.jpg)