Session Information
Date: Tuesday, November 10, 2015
Title: Fibromyalgia, Soft Tissue Disorders, Regional and Specific Clinical Pain Syndromes Poster II
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
The 2011 Fibromyalgia (FM) Survey Criteria is comprised of
measures of widespread pain (0-19 body areas) and 6 co-morbid symptoms (scored
0-12). The measure has been termed “fibromyalgianess,” which Wolfe and others have shown to be
predictive of pain and disability in many rheumatic disorders. We have shown that the continuous measure is
predictive of decreased opioid responsiveness and decreased improvement in pain
following arthroplasty, which we feel serves as a
marker of pain centralization. We sought
to develop a new scoring method for the items that would improve the predictive
utility of the measure for centralized pain by re-weighting the existing items
to better predict failure to improve pain following arthroplasty. Methods:
457 knee or hip arthroplasty
patients were included. Changes in pain (WOMAC and Brief Pain Inventory (BPI))
from baseline to 6 months post-surgery and patient global impression of change
(PGIC) served as outcomes. A count of 7 body zones (head, spine, trunk, arms,
and legs) was compared to the count of 19 original body areas in 6 regression
models. Multiple regression models with the criteria items as predictors were
then conducted to determine item importance. 10-fold cross validation was used.
A new scale score was formed by weighting individual items that consistently
best predicted surgical outcomes. Scale
scores were evaluated as predictors of surgical outcomes in regression models
in the development sample and a model testing sample of 391 other knee or hip arthroplasty patients. Performance was assessed with
R-square and AUROC. Results:
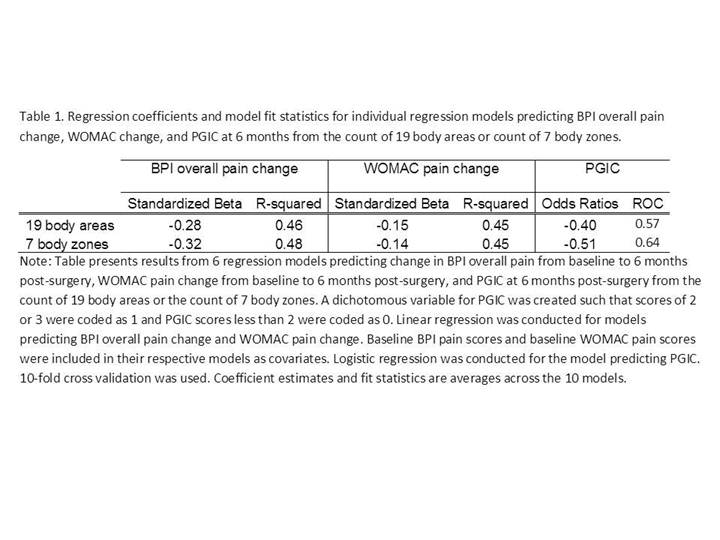
The count of 7 body zones performed as well or better than
the original 19 body areas (Table 1).
Thus the scoring using these zones was used in subsequent analyses. In
regression models, the body zones, headache, and trouble thinking were
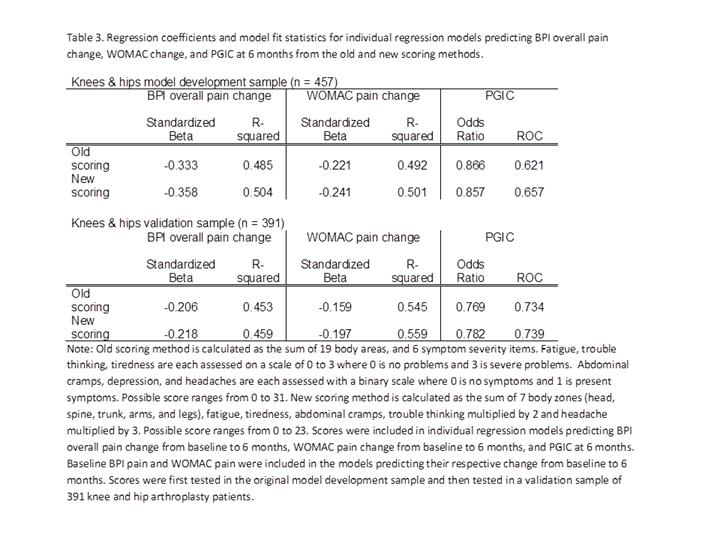
consistently strong predictors of outcomes (Fig 1). The new scale score in
which headache and trouble thinking were weighted 2X and 3X times,
respectively, outperformed the original FM measure (Table 3). Conclusion:
This work provides evidence that the original FM Survey
Criteria can be re-scored to be a better predictor of poor response to arthroplasty. Future
work is needed to determine the optimal manner to measure critical elements of
FM to define a new measure of pain centralization.
To cite this abstract in AMA style:
Moser S, Brummett C, Tsodikov A, Williams DA, Clauw DJ. Re-Scoring the 2011 Fibromyalgia Survey Criteria to be a Better Surrogate for Pain Centralization [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/re-scoring-the-2011-fibromyalgia-survey-criteria-to-be-a-better-surrogate-for-pain-centralization/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/re-scoring-the-2011-fibromyalgia-survey-criteria-to-be-a-better-surrogate-for-pain-centralization/