Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Depression is common among PsA patients, but rates may differ by PsA treatment. Our study compares rates of treated depression by PsA treatment type in patients with PsA.
Methods: We conducted a population-based cohort study of treated PsA patients in the MarketScan database in 2014-2018. Cohort entry was the date of first prescription for a study drug (apremilast, tumor necrosis factor inhibitor [TNF-i] biologics, interleukin-17 or -12/23 inhibitor [IL-i] biologics, conventional DMARDs, or systemic corticosteroids) after March 21, 2014, the date on which apremilast was approved. Patients with antidepressant treatment before cohort entry were considered prevalent cases and excluded. A patient was considered currently exposed to a study drug from the prescription date through the prescription duration + 30 days. Patients were followed from cohort entry through censor date, defined as the first of the following: index date (date patient became a case), end of record, or end of study period (October 31, 2018). Cases were patients with a first diagnosis of treated depression (without concurrent anxiety), defined as a diagnosis of depression with a prescription for an antidepressant within 30 days, and at least 7 days after cohort entry. We calculated incidence rates (IRs) and 95% confidence intervals (CIs) for each outcome per 1,000 patient-years among patients with at least 1 year of recorded medical history before their cohort entry date. Past use is defined as any days after the end of “current use” and before a new study drug prescription.
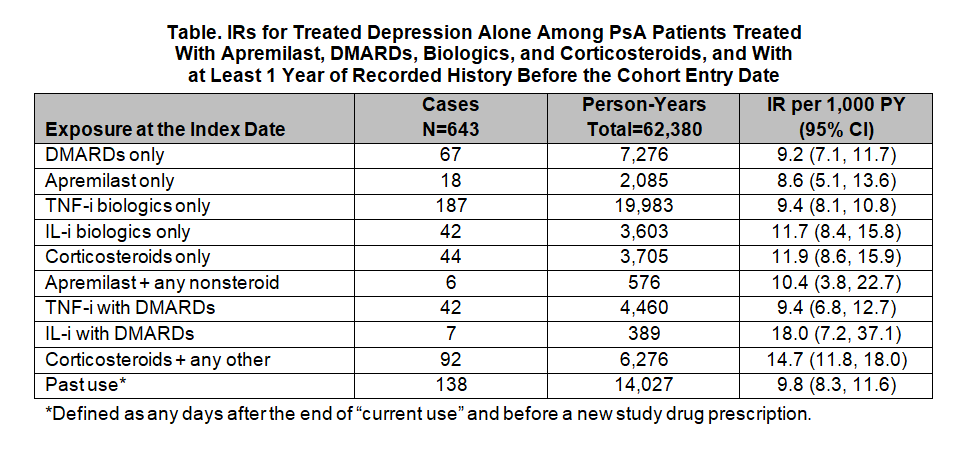
Results: The study population included 31,720 patients (median age at cohort entry: 53 years; female: 44%; history of depression: 4.8%). The IRs were similar between treatments with somewhat higher rates of treated depression among patients exposed to corticosteroids alone or in combination with any other study drug and patients exposed to IL-i alone or with DMARDs. Among the 643 treated depression cases, IRs (95% CIs) were as follows: apremilast only, 8.6 (5.1, 13.6); DMARDs only, 9.2 (7.1, 11.7); TNF-i only, 9.4 (8.1, 10.8); IL-i only, 11.7 (8.4, 15.8); and corticosteroids only, 11.9 (8.6, 15.9). The IRs for combination treatments were: TNF-i with DMARDs, 9.4 (6.8, 12.7); past use, 9.8 (8.3, 11.6); apremilast + other non-steroid study drugs, 10.4 (3.8, 22.7); corticosteroid + any other study drug, 14.7 (11.8, 18.0); and IL-i with DMARDs, 18.0 (7.2, 37.1).
Conclusion: Rates of treated depression were similar across PsA treatments, with somewhat higher rates of treated depression among patients exposed to corticosteroids alone or in combination with any other study drug and among patients exposed to IL-i alone or in combination with DMARDs.
To cite this abstract in AMA style:
Vasilakis-Scaramozza C, Persson R, Wilcox Hagberg K, Niemcryk S, Peng M, Paris M, Lindholm A, Jick S. Rates of Treated Depression Among Patients with Psoriatic Arthritis Treated with Apremilast, Biologics, DMARDs, and Corticosteroids in the US MarketScan Database [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/rates-of-treated-depression-among-patients-with-psoriatic-arthritis-treated-with-apremilast-biologics-dmards-and-corticosteroids-in-the-us-marketscan-database/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rates-of-treated-depression-among-patients-with-psoriatic-arthritis-treated-with-apremilast-biologics-dmards-and-corticosteroids-in-the-us-marketscan-database/