Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
There are unmet needs for patients (pts) suffering from rheumatoid arthritis (RA) in China, where a limited number of anti-TNFs are available.1, 2 Certolizumab pegol (CZP), with its demonstrated efficacy and safety, would provide an additional option for Chinese RA pts.3, 4 We report results from RAPID-C, which assessed the efficacy and safety of CZP in combination with methotrexate (MTX) in Chinese pts with active RA and an inadequate response to MTX.
Methods:
RAPID-C (NCT02151851) was a 24-week (wk), phase 3, double-blind, placebo-controlled study conducted in 30 centers across China. Pts with active RA were randomized 3:1 to receive CZP or placebo (PBO), together with MTX. Pts received either loading doses of CZP 400 mg or PBO at Wks 0, 2 and 4, followed by CZP 200 mg or PBO every 2 wks thereafter. The primary endpoint was ACR20 at Wk24, analyzed using logistic regression in the full analysis set (FAS) with non-responder imputation for missing data. Secondary endpoints were ACR50/70 responses and physical function as measured by change from baseline (CFB) in HAQ-DI at Wk24. ACR core components, CRP, DAS28(ESR) and fatigue (measured using BRAF-MDQ) were assessed at selected study visits. CFB in HAQ-DI and other continuous variables were analyzed in the FAS using analysis of covariance with last observation carried forward imputation for missing data. Incidence of treatment-emergent adverse events (TEAEs) and other safety parameters were monitored over the treatment period.
Results:
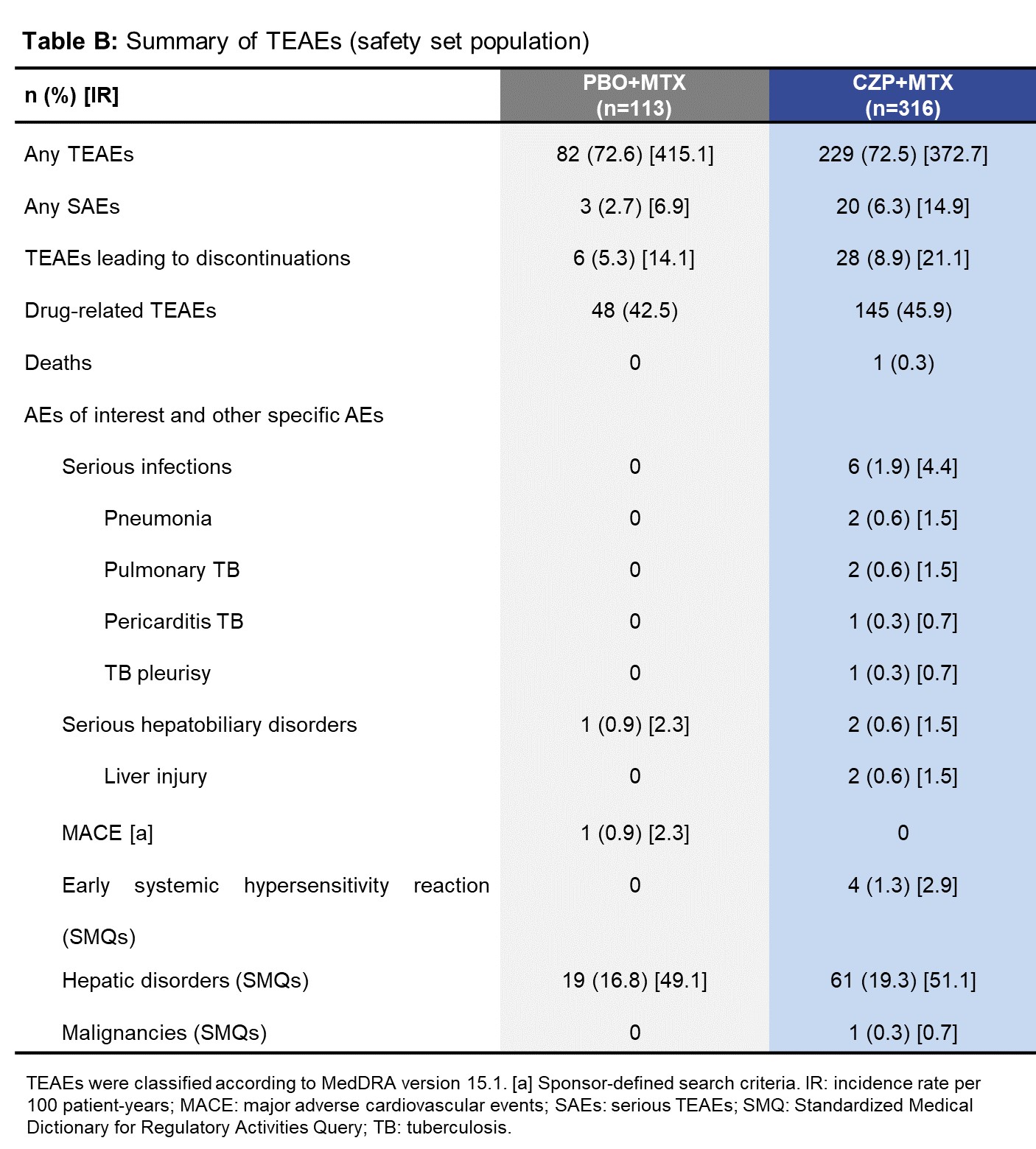
The FAS included 312 CZP+MTX pts and 113 PBO+MTX pts. The demographic and baseline disease characteristics were balanced between treatment arms. Compared with PBO+MTX, a significantly higher percentage of CZP+MTX pts achieved ACR20 response at Wk24 (54.8% vs 23.9%; odds ratio: 3.9, p<0.001). Greater improvements in HAQ-DI, higher ACR50/70 responses (Table A) and higher DAS28 remission rates were also observed in CZP+MTX pts at Wk24. Rapid onset of response was observed as early as Wk1 for most efficacy outcomes in CZP+MTX pts. The safety profile of CZP was in line with previous CZP studies (Table B).3, 4 No new safety signals were identified in this population of Chinese pts.
Conclusion:
CZP in combination with MTX has shown an acceptable safety profile, a rapid onset of action and sustained effects in reducing the signs and symptoms of RA and improving physical function in Chinese RA pts with an inadequate response to MTX.
*These authors contributed equally to this work.
References:
1. An Y. Clin Rheumatol 2017;36:35-43
2. Wang GY. Clin Rheumatol 2015;34:221-30
3. Keystone E. Arthritis Rheum 2008;58:3319-29
4. Smolen J. Ann Rheum Dis 2009;68:797–804
To cite this abstract in AMA style:
Bi L, Li Y, He L, Xu H, Gu J, Wang G, Zhang Z, Liu Y, Boehnlein M, Dunkel J, Shao J, Harris K, Li Z. Rapid Onset of Response Observed with Certolizumab Pegol in Rheumatoid Arthritis Patients with Inadequate Response to Methotrexate: Efficacy and Safety Results of a Randomized, Double-Blind, Placebo-Controlled Phase 3 Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/rapid-onset-of-response-observed-with-certolizumab-pegol-in-rheumatoid-arthritis-patients-with-inadequate-response-to-methotrexate-efficacy-and-safety-results-of-a-randomized-double-blind-placebo-c/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rapid-onset-of-response-observed-with-certolizumab-pegol-in-rheumatoid-arthritis-patients-with-inadequate-response-to-methotrexate-efficacy-and-safety-results-of-a-randomized-double-blind-placebo-c/