Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Best practice for the use of immunosuppression to optimize vaccine response remains uncertain. We conducted the Covid VaccinE Response (COVER) trial, a multicenter, randomized controlled platform trial to assess the response to the SARS-CoV-2 booster in rheumatology patients on immunomodulatory therapies. The primary goal was to assess whether randomizing to hold Janus kinase inhibitors (JAKi), interleukin-17 inhibitors (IL-17i), abatacept (ABA), or tumor necrosis factor inhibitors (TNFi) for 2 weeks at the time of COVID-19 booster yields improved vaccine response compared to continuing therapy. Our previous report (ACR Convergence 2023) found no significant benefit from holding ABA or TNFi for 2 weeks but did observe a diminished antibody response to COVID boosting in ABA patients.
Methods: COVER enrolled patients with rheumatoid arthritis (RA) or spondyloarthritis (SpA) being treated with JAKi/biologics in the Excellence Network in RheumatoloGY (ENRGY), a practice-based research network of US rheumatologists. Patients, who previously received >2-dose mRNA vaccines, were randomized (1:1) to continue or hold therapy for 2 weeks following a COVID-19 booster. LabCorp Cov2Quant IgG™ anti-Spike assay measured anti-Receptor Binding Domain (RBD) IgG antibodies before and 6 + 2 weeks after the booster dose. We compared mean log-transformed differences pre- and post-booster and report the geometric mean fold rise (GMFR) by intervention group and therapy. We used linear regression, including all study arms (ABA and TNFi), to compare groups for change in COVID-19 RBD antibody levels, adjusting for age, sex, BMI, methotrexate use, steroid use, and time from booster to measurement. Patient-reported flare following booster was compared by intervention group.
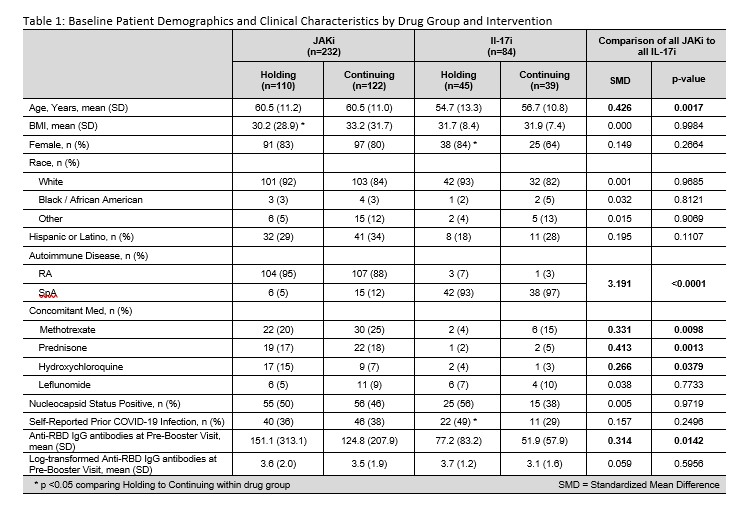
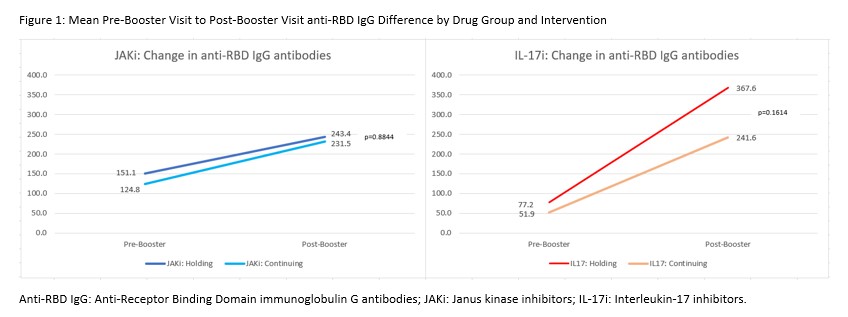
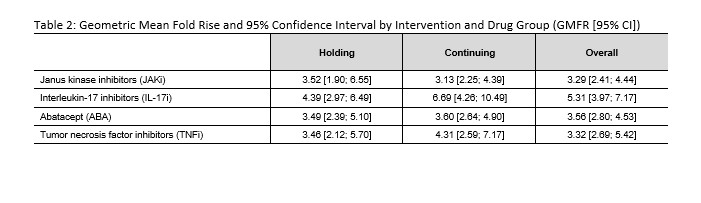
Results: Patients on JAKi (n=232) or IL-17i (n=121) were 79% female, 88% white, 29% Hispanic, with mean (SD) age of 59.2 (11.6) years and BMI of 31.8 (7.6) kg/m2 (Table 1). Report of flare following booster was greater among those holding therapy for 2 weeks (JAKI: 34% in hold group vs 9% continue, p< 0.0001; IL-17i: 30% vs 16%, p=0.20). Following vaccine booster dose, RBD antibody titers increased significantly in both JAKi and IL-17i groups (p< 0.0001 for all arms) (Figure 1) and all groups showed a GMFR >3 (Table 2). Mean increase in RBD antibodies in the hold vs. continue arm was not significantly different by drug group, before or after adjustment. A decreased effect in change in RBD antibodies was seen for JAKi (β=-0.42, p=0.018) and ABA (β=-0.73, p< 0.001), referent to IL-17i, as well as steroid use (β=-0.31, p=0.041); higher BMI (β=0.015, p=0.039) was associated with greater change in antibody levels.
Conclusion: RA and SpA patients taking JAKi or IL-17i had a significant increase in humoral antibody response after receiving a COVID-19 booster and holding therapy for 2 weeks did not enhance response compared to continuing therapy. As previously observed with ABA, use of JAKi blunted COVID-19 vaccine responses. Based on these interim results and an increase in the frequency of disease flare, interrupting JAKi or IL-17i at the time of COVID-19 boosting does not appear warranted. Work is ongoing to evaluate response based on neutralizing antibodies.
To cite this abstract in AMA style:
Mudano A, Cutter G, Holladay E, Winthrop K, Mikuls T, Thiele G, Law M, Bastidas M, Zikry M, Chun K, George M, Sinha J, Alvarez N, Tesser J, Curtis J. Randomization to Holding versus Continuing (JAKi, IL17) and Autoimmune Patient Responses to COVID-19 Boosters: Results from the Covid-19 VaccinE Response in Rheumatology Patients (COVER) Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/randomization-to-holding-versus-continuing-jaki-il17-and-autoimmune-patient-responses-to-covid-19-boosters-results-from-the-covid-19-vaccine-response-in-rheumatology-patients-cover-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/randomization-to-holding-versus-continuing-jaki-il17-and-autoimmune-patient-responses-to-covid-19-boosters-results-from-the-covid-19-vaccine-response-in-rheumatology-patients-cover-study/