Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
TNF inhibitors (TNFi) have proven to be effective in the treatment of rheumatoid arthritis (RA). They are however associated with side effects and high costs, making dose reduction or discontinuation an attractive option. The primary aim of this study (DRESS) was to assess non-inferiority with regard to persistent disease worsening (flare) between a TNFi dose reduction strategy and usual care in daily clinical practice.
Methods
Patients with RA and low disease activity using adalimumab or etanercept were randomised (2:1) to a dose reduction strategy or usual care, both in tight control setting. The TNFi dose reduction strategy consisted of stepwise increasing the interval between injections every 3 months until flare or discontinuation.1 In case of flare, the TNFi was restarted or escalated. A flare was defined as DAS28-CRP increase >1.2 or DAS28-CRP increase >0.6 and current DAS28-CRP ≥3.2, compared to baseline DAS28-CRP.2 A persistent flare was defined as a flare duration ≥ 12 weeks. During 18 months follow up, data were collected on DAS28-CRP, HAQDI, EQ-5D, RA medication use, and costs. The primary outcome was the difference in proportions of patients with persistent flare between the two groups compared against a non-inferiority (NI) margin of 20%.
Results
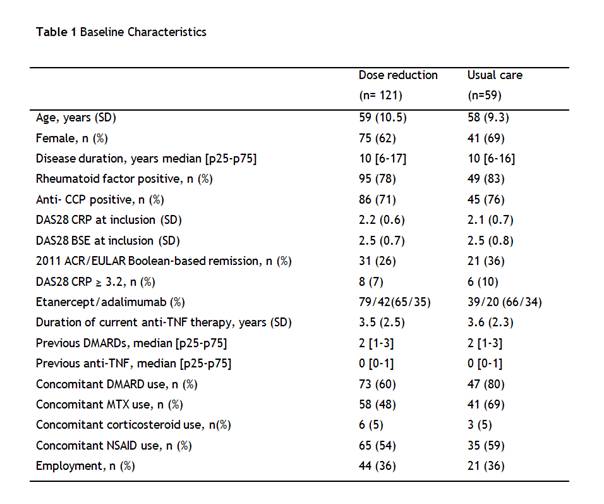
180 patients were included (table 1). Cumulative incidence of persistent DAS28-CRP flare was not significantly higher in the dose reduction group compared to the usual care group, 10% versus 12% of patients respectively (difference 2%, 95% CI -10 to 12), the upper limit of the 95% CI being clearly below the NI margin. Mean DAS28-CRP remained low, with only at 9 months follow up a significant, but small, difference between groups (figure 1A). HAQ scores remained stable in both groups (figure 1B) as did quality of life (figure 1C). In the dose reduction group, the TNFi could successfully be stopped at 18 months in 20% (95% CI 13 to 28) of patients, the interval successfully increased in 43% (95% CI 34 to 53) and in 37% (95% CI 28 to 46) of patients no dose reduction was possible. Incidence and nature of serious adverse events were similar between groups. Costs were significantly lower in the dose reduction group (mean difference per patient €9k).
Conclusion
A simple tight control TNFi dose reduction strategy has been shown to be non-inferior to usual care in maintaining disease control, function and quality of life, while reducing exposition to TNFi and costs.
References
1: den Broeder et al. BMC Musculoskelet Disord. 2013 24;14:299
2: van der Maas et al. Ann Rheum Dis. 2013;72(11):1800-5.
Disclosure:
N. van Herwaarden,
None;
A. van der Maas,
Roche, MSD, ,
9;
M. Minten,
None;
F. H. J. van den Hoogen,
None;
R. F. van Vollenhoven,
Abbott, BMS, GSK, MSD, Pfizer, Roche, UCB ,
2,
Abbott, BMS, GSK, MSD, Pfizer, Roche, UCB ,
5;
J. W. J. Bijlsma,
AbbVie, Roche, Pfizer, MSD, UCB, BMS,
2,
AbbVie, Roche, Pfizer, MSD, UCB, BMS, Jansen,
5;
B. van den Bemt,
Roche Pharmaceuticals, Pfizer,
2,
Roche, Pfizer, MSD Abbvie,
5;
A. A. den Broeder,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/randomised-controlled-non-inferiority-study-of-dose-reduction-and-withdrawal-of-adalimumab-and-etanercept-in-rheumatoid-arthritis/