Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Ixekizumab (IXE), an anti-interleukin-17A monoclonal antibody, was shown to be superior to placebo (PBO) in clinical responses and inhibiting the progression of structural joint damage in patients with PsA treated for 24 weeks (wks).1 The objective was to assess the progression of structural joint damage in PsA pts with IXE for up to 52 wks.
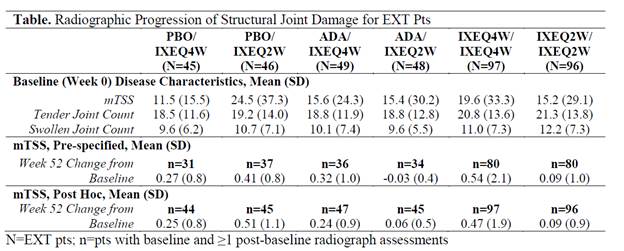
Methods: Biologic DMARD-naïve pts with active PsA (N=417) entered into SPIRIT-P1 (NCT01695239), a double-blind phase 3 trial. Pts must have had ≥1 joint erosion on the hand and foot x-rays confirmed by central reading or had a C-reactive protein level >6 mg/L at screening. 417 pts were randomized to IXE 80 mg every 2 wks (Q2W; N=103) or 4 wks (Q4W; N=107) following a 160 mg initial dose, PBO (N=106), or adalimumab 40 mg every 2 wks (ADA; active reference arm; N=101) for 24 wks. In the Extension period (EXT: Wks 24-52), PBO and ADA pts were re-randomized (1:1) to IXEQ2W or IXEQ4W at Wk 16 (inadequate responders) or Wk 24; ADA pts underwent a washout prior to IXE treatment. All pts were assessed for structural joint damage using the van der Heijde-modified PsA Total Sharp Score (mTSS, 0-528 scale). X-rays at Wks 0, 24, and 52 were scored independently by 2 readers blinded to time point and clinical data (average of readers). mTSS was excluded from the pre-specified analysis if the radiograph was taken after the scheduled visit date. In a post hoc analysis, mTSS from a radiograph taken after the scheduled visit date was interpolated and considered as observed data. Any missing data at Wk 52, in either presentation, were imputed using a linear extrapolation if they had at least 1 post-baseline value.
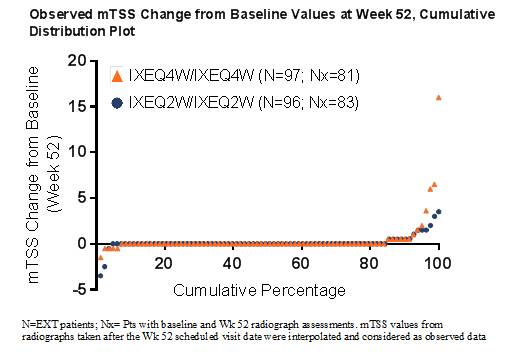
Results: Pts had active PsA at Wk 0 (Table). 381 pts (91.3%) entered the EXT, with 374 (98.2%) having radiographs collected during the EXT. Wk 52 mean (SD) mTSS change from baseline were 0.54 (2.11) and 0.09 (1.0) for pts randomized to IXEQ4W and IXEQ2W at baseline, respectively. Similar changes at Wk 52 were obtained with the post hoc analysis (Table). The majority of IXEQ2W or IXEQ4W pts exhibited no structural progression through 1 year of IXE treatment (Figure). In pts who switched from PBO or ADA to IXE, Wk 52 mean change from baseline mTSS values scores ranged from -0.03 to 0.41 (Table).
Conclusion: Over a 52-wk period, minimal changes in mTSS were observed in PsA pts entering the EXT and treated with IXEQ2W or IXEQ4W.
1. Mease P et al. 2017 ARD 76(1):79
To cite this abstract in AMA style:
van der Heijde D, Okada M, Lee CH, Shuler C, Rathmann S, Lin CY, Mease PJ. Radiographic Progression of Structural Joint Damage in Patients with Active Psoriatic Arthritis Treated with Ixekizumab over 52 Weeks [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/radiographic-progression-of-structural-joint-damage-in-patients-with-active-psoriatic-arthritis-treated-with-ixekizumab-over-52-weeks/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/radiographic-progression-of-structural-joint-damage-in-patients-with-active-psoriatic-arthritis-treated-with-ixekizumab-over-52-weeks/