Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Knee osteoarthritis (OA) is characterized by pain, functional impairment, disability, and joint space narrowing due to degradation of articular cartilage and bone remodeling. The Wnt signaling pathway is known to play a central role in the formation of joint tissues, and altered Wnt signaling has been associated with cartilage loss in preclinical and clinical studies.1 SM04690 is a small molecule inhibitor of the Wnt pathway in development as a potential OA therapeutic to be administered as an intra-articular (IA) injection into the affected joint. A phase 1, first-in-human, multicenter, 24-week, single-dose-escalation, randomized controlled trial (RCT) of SM04690 was completed in subjects with moderate to severe knee OA. This report provides exploratory radiographic outcomes.
Methods: In the completed phase 1 RCT, escalation cohorts were dosed at 0.03 mg, 0.07 mg, and 0.23 mg SM04690 per 2 mL injection. A sample size of 20 subjects (randomized, 16 active: 4 placebo) per dosing cohort was selected. Subjects were administered a single IA injection into the target knee on Treatment Day 1, and participated in a follow-up period of 24 weeks. Safety, pharmacokinetics (PK), biomarker, and preliminary efficacy data were collected. To evaluate change from baseline in joint space width (JSW), X-rays of the target knee joint were taken at baseline and week 24. An exploratory analysis of change in JSW was conducted using repeated measures analysis of covariance (ANCOVA) adjusting for baseline JSW in the modified Intention-to-treat (mITT) population.
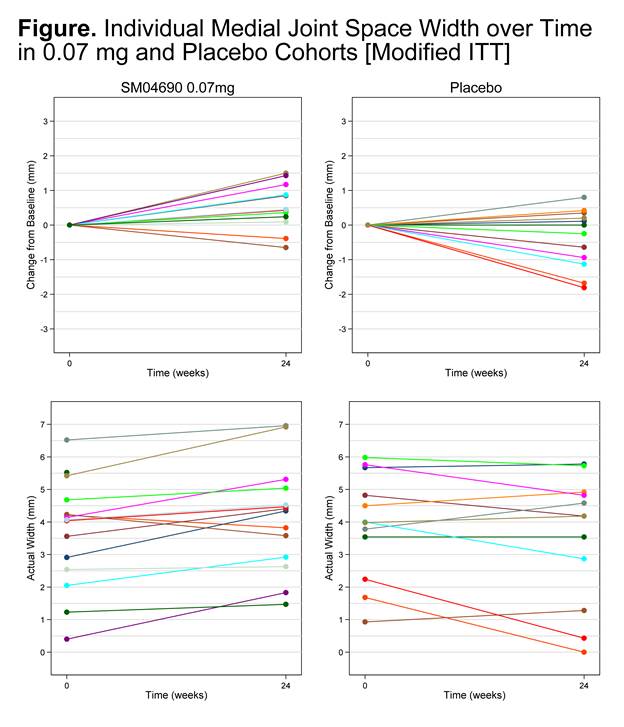
Results: Sixty-one subjects (average age 62.6 [±5.7] years, female n=41 [67%], average BMI 30.4 [±4.7] kg/m2) were enrolled. In the mITT population at 24 weeks, the 0.07 mg cohort displayed a statistically significant change from baseline in medial JSW (0.49 mm [0.75]) as compared to placebo (-0.33 mm [0.87]) (P=0.02). No change or improvement in medial JSW was observed in 9 of 15 subjects (60%) in the 0.03 mg cohort, 12 of 15 subjects (80%) in the 0.07 mg cohort, and 6 of 11 (55%) subjects in the placebo cohort. In the 0.23 mg cohort, 9 of 16 (56%) subjects demonstrated worsening of JSW. Individual radiographic responses for the 0.07mg cohort and placebo group are presented in the Figure.
Conclusion: Exploratory radiographic outcomes from this completed phase 1 study suggested that treatment with SM04690 may have maintained or increased joint space width compared to placebo, providing proof of concept data of efficacy in the treatment of knee OA. Further evaluation studies are ongoing. References:
1. Gelse K. Osteoarthr Cartil. 2012; 20(2):16271
To cite this abstract in AMA style:
Swearingen CJ, Majumdar S, Simsek I, DiFrancesco A, Tambiah J, Yazici Y. Radiographic Outcomes from a Randomized, Double-Blind, Placebo-Controlled, Phase 1 Study of a Novel, Intra-Articular, Injectable, Wnt Inhibitor (SM04690) in the Treatment of Osteoarthritis of the Knee [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/radiographic-outcomes-from-a-randomized-double-blind-placebo-controlled-phase-1-study-of-a-novel-intra-articular-injectable-wnt-inhibitor-sm04690-in-the-treatment-of-osteoarthritis-of-the-knee/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/radiographic-outcomes-from-a-randomized-double-blind-placebo-controlled-phase-1-study-of-a-novel-intra-articular-injectable-wnt-inhibitor-sm04690-in-the-treatment-of-osteoarthritis-of-the-knee/