Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Fibromyalgia & Other Clinical Pain Syndromes (0474–0477)
Session Type: Abstract Session
Session Time: 11:15AM-11:30AM
Background/Purpose: Fibromyalgia is a chronic pain syndrome characterized by widespread musculoskeletal pain, mood disorders, fatigue, and cognitive and sleep disturbance. Mainstay treatments include tricyclic antidepressants, selective serotonin/serotonin norepinephrine reuptake inhibitors, and antiepileptic drugs. The purpose of this study was to examine whether or not gender and racial disparities exist in the context of research subjects recruited for clinical studies performed on the only three FDA approved medications for fibromyalgia.
Methods: The PubMed database was searched for clinical trials studying duloxetine (DLX), milnacipran (MLN), or pregabalin (PRG) for the treatment of fibromyalgia. Studies with randomized, double blind, placebo controlled design involving any of the above medications as monotherapy and reporting on gender and ethnic demographic parameters published over the last 10 years were included. Ethnic backgrounds were grouped into Caucasian or White, African American, Hispanic, Asian, and Other respectively.
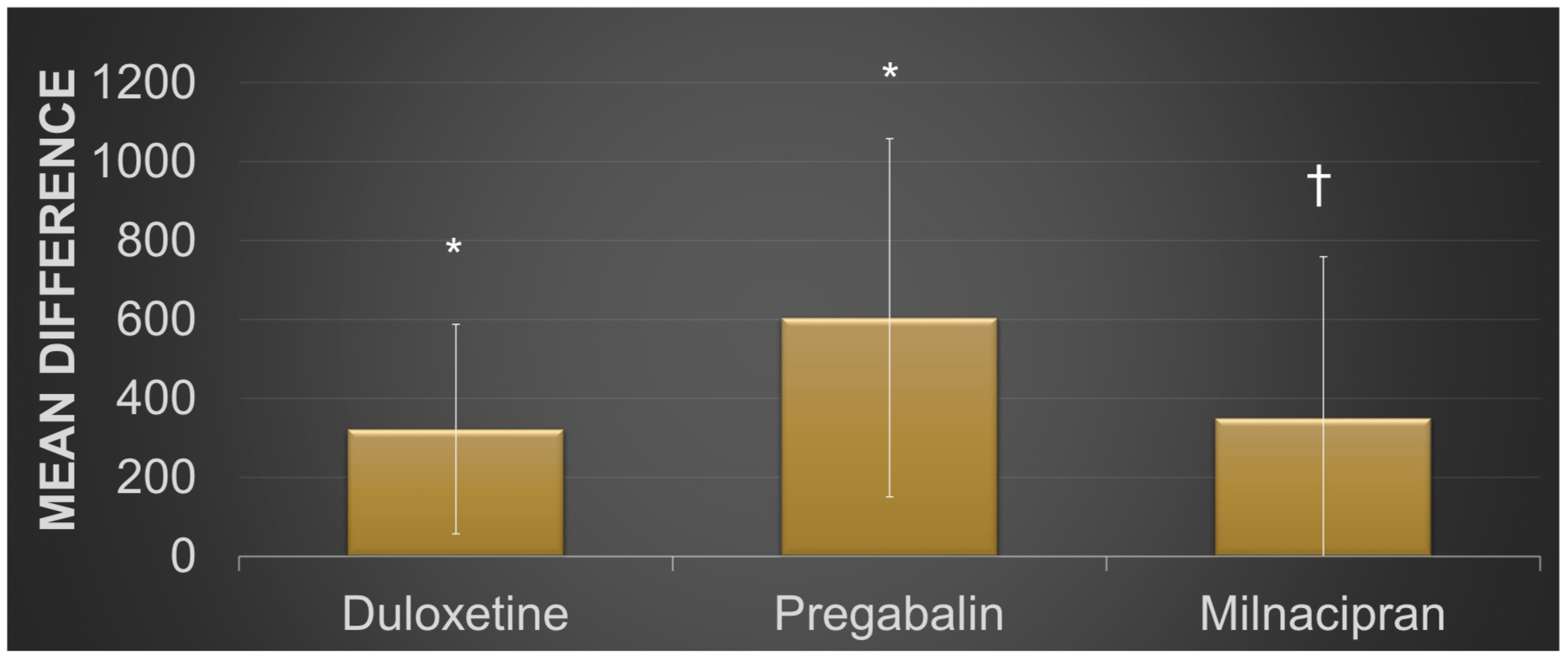
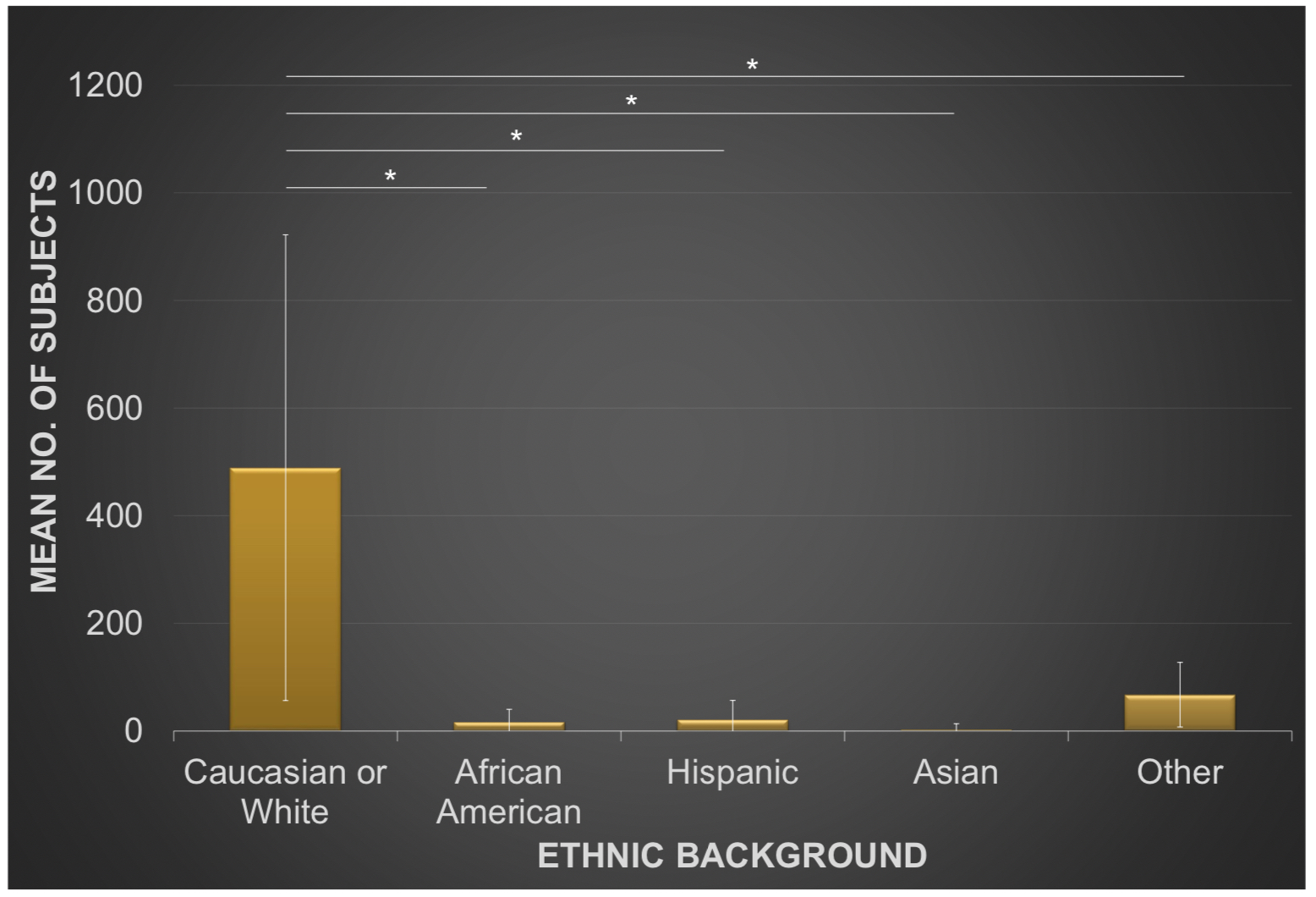
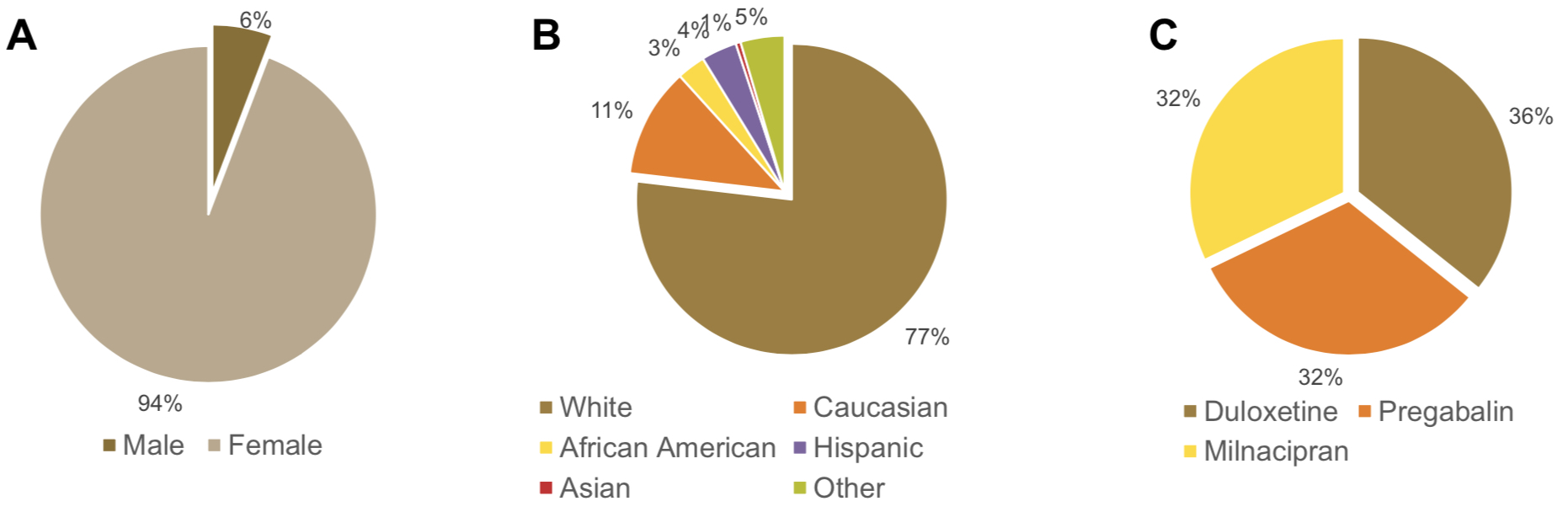
Results: A total of 28 studies met inclusion criteria. Paired t-tests demonstrated a female gender preponderance of research participants for all of the DLX, MLN, and PRG treatment groups analyzed separately (p< 0.05 each) and collectively (p< 0.001). White or Caucasian was the predominant ethnic group across all treatment groups analyzed separately (86.4±6.1% DLX, 89.2±6.2% PRG, and 87.3±8.1% MLN) or collectively (87.6±6.7%). There were no statistically significant differences between group means across all treatment groups as determined by one-way ANOVA for White or Caucasian (F(2,25)=1.57, p=0.23), African American (F(2,25)=2.71, p=0.09), Asian (F(2,25)=0.64, p=0.54), or all ethnic groups combined (F(2,25)=1.6, p=0.22); as was the case for studies conducted within (F(2,22)=1.79, p=0.19) or outside (F(1,3)=0.1, p=0.77) the USA. Nonetheless, a statistically significant difference was observed by one-way ANOVA for the Hispanic ethnic group (F(2,25)=5.19, p< 0.05). Paired t-tests revealed a statistically significant overrepresentation of Caucasian or White research subjects (489.25±432.70) in comparison to African American (17±23.39, t(27)=6.06, p< 0.001), Hispanic (21.29±35.43, t(27)=5.78, p< 0.001), Asian (2.93±10.51, t(27)=5.95, p< 0.001), or all other ethnic groups examined (67.32±60.03, t(27)=5.76, p< 0.001). A similar pattern of Caucasian or White subject overrepresentation in comparison to all other ethnic groups was found on subgroup analysis for all of the DLX (mean(diff)=322.20±265.27, t(9)=3.83, p< 0.005), PRG (mean(diff)=604.56±453.60, t(8)=3.40, p< 0.005), and MLN (mean(diff)=350.11±408.85, t(8)=2.57, p< 0.05) treatment groups respectively.
Conclusion: Evidence of widespread gender and racial disparities amongst subjects recruited for clinical trials of various pharmacologic agents for fibromyalgia exist. Whereas female preponderance for fibromyalgia is believed to reflect a true gender predilection of this disorder more research is needed to determine whether or not racial disparities observed indeed reflect on the nature of the disease.
To cite this abstract in AMA style:
Diab R, Malik A, Al Rifai M, Ang D. Quantitative Analysis of Gender and Racial Disparities in Randomized Controlled Trials of Fibromyalgia [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/quantitative-analysis-of-gender-and-racial-disparities-in-randomized-controlled-trials-of-fibromyalgia/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantitative-analysis-of-gender-and-racial-disparities-in-randomized-controlled-trials-of-fibromyalgia/