Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Glucocorticoids (GCs) are frequently and often chronically used for the treatment of rheumatoid arthritis (RA) and other immune diseases and vasculitis. An estimated 0.8–1.2% of the population in developed countries at any moment in time uses GCs. EULAR guidelines state that the adverse effects (AEs) of GC therapy should be considered and discussed with the patient before treatment is initiated.1 However, reliable quantitative data on cutaneous AEs of low-to medium dose GC’s are lacking.
Methods: We performed a study to assess the occurrence of cutaneous AEs of GCs and its association with current and cumulative GC-doses. This cross-sectional study was performed at the outpatient departments of the Charité University Medicine Berlin and the University Medical Center Utrecht; 381 RA-patients were included. Patients were classed into 4 groups, according their mean daily dose during the past 12 months: 0 mg (n=87), <5mg (n=108), 5–7.5 mg (n=130), and >7.5 mg (n=56) of prednisolone equivalent. Cushingoid appearance, ecchymosis, stretch marks, steroid acne, perioral dermatitis, hirsutism, and scalp hair loss were assessed by physical examination using a predefined scoring system, and by patients’ self-assessments. Easy bruising, skin atrophy, and impaired wound healing were patients’ self-assessed reports. Data were analyzed according GC dose categories and cumulative doses.
Results: Of the 381 patients, 76% was female; 67% was rheumatoid factor positive and 55% had erosive joint disease. The median (quartiles) disease duration was 9 (4-17) years. The mean number of lifetime conventional synthetic DMARDs used was 2.6 and for biological DMARDs it was 1.0. The median (quartiles) total duration of GC use was 4.0 (1.5-10) years, and the mean (sd) cumulative dose was 14 (17) g. Cushingoid habitus, easy bruising, skin atrophy, and impaired wound healing as reported by patients occurred significantly more frequent in those using GC the past 12 months, compared to those not using GC, see figure. At physicians’ assessments, only Cushingoid habitus and ecchymosis were more prevalent in GC-users, see figure. The prevalence of these AEs was statistically significantly positively associated with current and cumulative GC dose. There was a low occurrence of abnormal stretch marks, acne, perioral dermatitis, alopecia, and hirsutism in our study, and there were no correlations between these AEs and GC therapy.
Conclusion: Cushingoid habitus, easy bruising, skin atrophy, impairment of wound healing, and ecchymosis are AEs of GC which can be observed relatively frequently in clinical daily practice. They are GC dose-dependent (current and cumulative dose). At the lower GC doses used in RA, abnormal stretch marks, acne, perioral dermatitis, alopecia, and hirsutism are rare AEs of GC. 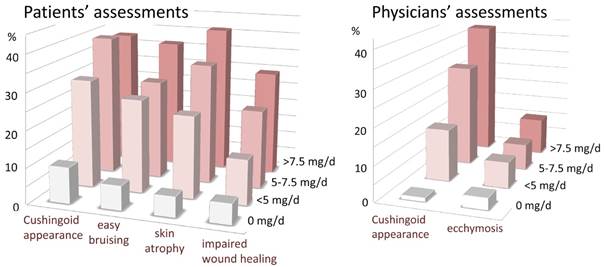
To cite this abstract in AMA style:
Buttgereit F, Amann J, Breitenfeldt F, Huscher D, Bijlsma JW, Jacobs JW. Quantification of Adverse Glucocorticoid Effects on Skin in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/quantification-of-adverse-glucocorticoid-effects-on-skin-in-rheumatoid-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/quantification-of-adverse-glucocorticoid-effects-on-skin-in-rheumatoid-arthritis/
