Session Information
Session Type: Abstract Session
Session Time: 2:00PM-3:30PM
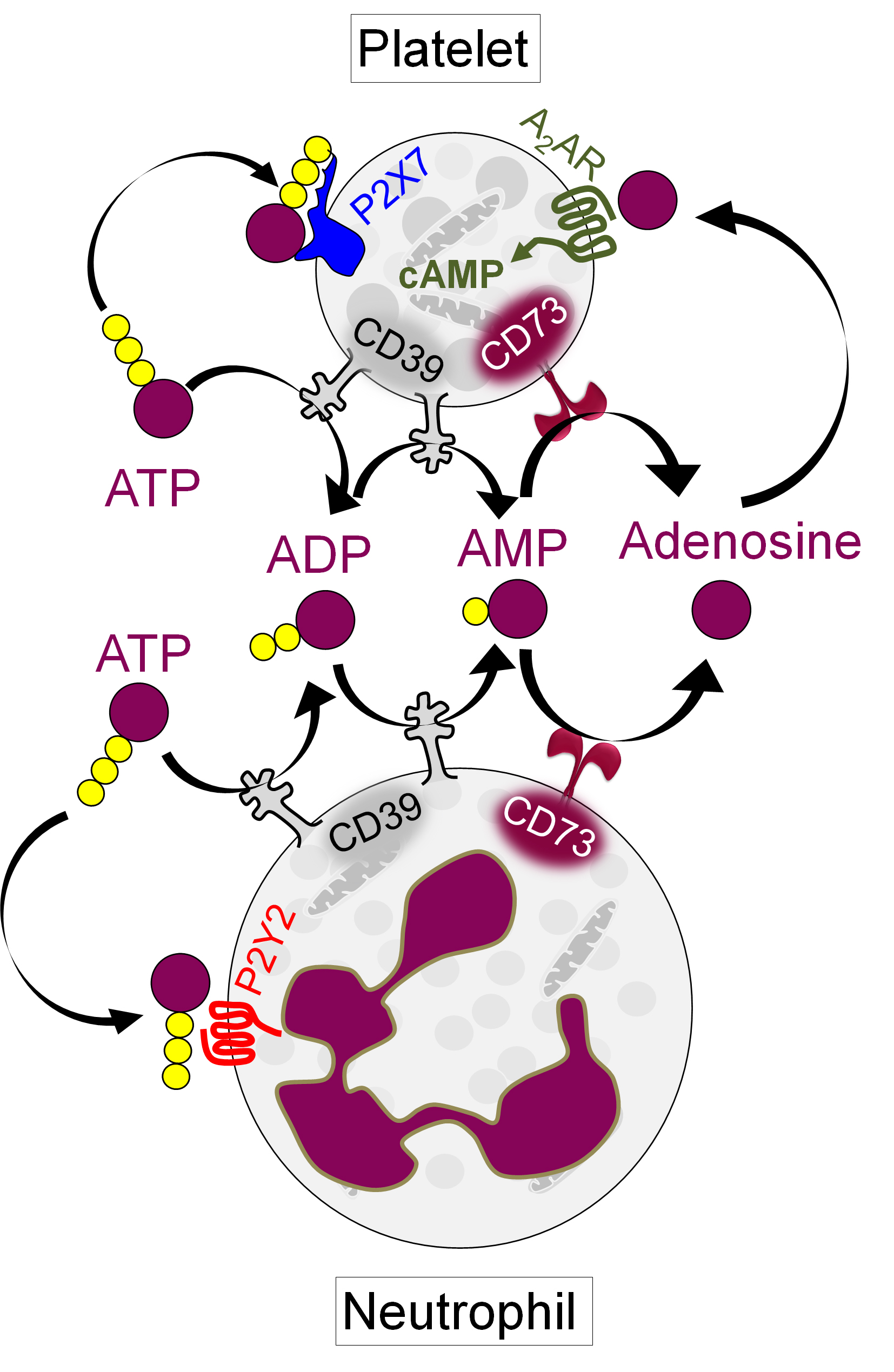
Background/Purpose: In first responding cells such as neutrophils and platelets, extracellular ATP released from activated or dying cells engages cell-surface receptors to launch proinflammatory and prothrombotic signals. As a counterpoint to this thromboinflammatory purinergic signaling, the surface ectonucleotidases CD39 (ATP to AMP) and CD73 (AMP to adenosine) convert ATP into homeostatic adenosine. Adenosine then activates G protein-coupled receptors to increase intracellular cyclic AMP (cAMP), thereby blunting inflammation and thrombosis. Here, we aimed to understand the potential relationship between the CD39/CD73 axis, neutrophils, and platelets in antiphospholipid syndrome (APS).
Methods: Neutrophil-platelet aggregates (NPAs) and platelet P-selectin (CD62P) were assessed in the blood of patients with primary APS by flow cytometry. In parallel, CD39 and CD73 activities on both neutrophils and platelets were measured by a standard malachite green assay. Levels of adenosine generation and intracellular cAMP were estimated using standard kits. In some experiments, healthy neutrophils and platelets were treated with APS IgG, specific CD39/CD73 activity inhibitors (ARL 67156, PSB 12379), or inhibitors of various surface receptors.
Results: As compared with healthy controls (n=48), patients with primary APS (n=55) showed at least a 50% reduction in median activities of neutrophil CD39 (p< 0.0001), neutrophil CD73 (p< 0.0001), platelet CD39 (p< 0.0001), and platelet CD73 (p< 0.0004). These changes were negatively correlated with significant increases in both NPAs (up to 80%, p< 0.0001) and platelet activation as defined by CD62P expression (p< 0.002). The levels of NPAs were higher in patients with APS who had a history of thrombosis than those without. When healthy neutrophils and platelets were cultured with either APS IgG or a CD39 inhibitor, there was a dose-dependent increase in NPA formation (from baseline 15% to 70%), and this phenotype was substantially blocked by inhibition of either the neutrophil P2Y2 receptor or the platelet P2X7 receptor. Focusing further on APS platelets, we found a significant decrease in their ability to generate adenosine (p< 0.01), as well as in their accumulation of intracellular cAMP (p< 0.001). Notably, CD62P expression was inversely correlated with platelet cAMP levels in patients (r=-0.52, p=0.007). Exposure of healthy platelets to APS IgG induced AKT phosphorylation (ser473) followed by downstream activation of GSK3β (ser9). This pro-activation signaling was efficiently blunted by agents that either activated adenosine receptors or directly boosted intracellular cAMP.
Conclusion: In primary APS, deficiency of CD39 and CD73 on both neutrophils and platelets potentiates pro-thrombotic platelet activation and NPA formation. By interrogating the downstream mechanisms, we identified several potential therapeutic targets including neutrophil P2Y2 and platelet P2X7 (Figure 1). Adenosine receptor agonists might be a strategy for restoring platelet homeostasis in APS. Overall, we speculate that a subset of patients with APS would benefit from therapies that modulate extracellular purinergic signaling.
To cite this abstract in AMA style:
Somanathapura N, Hoy C, Yalavarthi S, Sarosh C, De Moraes Mazetto Fonseca B, Ranger C, Rysenga C, Tambralli A, Madison J, Zuo Y, Knight J. Purinergic Signaling as a Potential Therapeutic Target for APS Thromboinflammation [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/purinergic-signaling-as-a-potential-therapeutic-target-for-aps-thromboinflammation/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/purinergic-signaling-as-a-potential-therapeutic-target-for-aps-thromboinflammation/