Session Information
Date: Sunday, November 10, 2019
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Patient Reported Outcomes
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient-reported outcome measures (PROMs) are often used to include the patients perspective on treatment (outcome). However, the choice of PROMs is difficult, as there are multiple PROMs available to measure a single construct. These PROMs differ in psychometric properties, length and scoring methods and often suffer from ceiling effects. To improve measurements the National Institute of Health (NIH) initiated the development of the Patient Reported Outcomes Measurement Information System (PROMIS®), which contains multiple item banks to assess different construct of Health-Related Quality of Life (HRQoL), symptoms and psychosocial functioning.The purpose of this study was to assess the psychometric properties of eight pediatric PROMIS item banks in a clinical sample of children with Juvenile Idiopathic Arthritis (JIA).
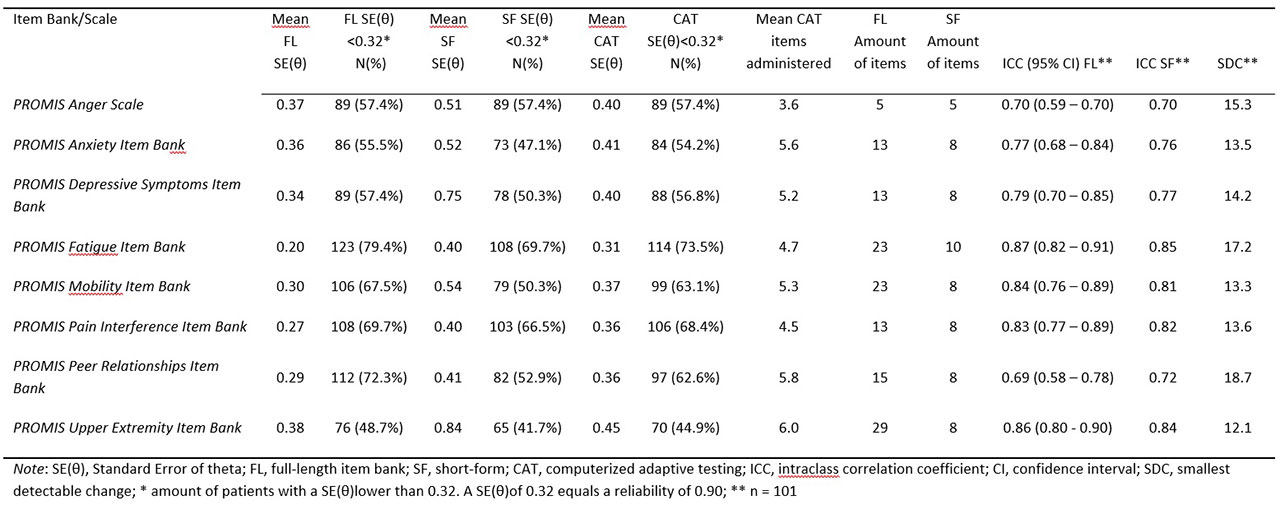
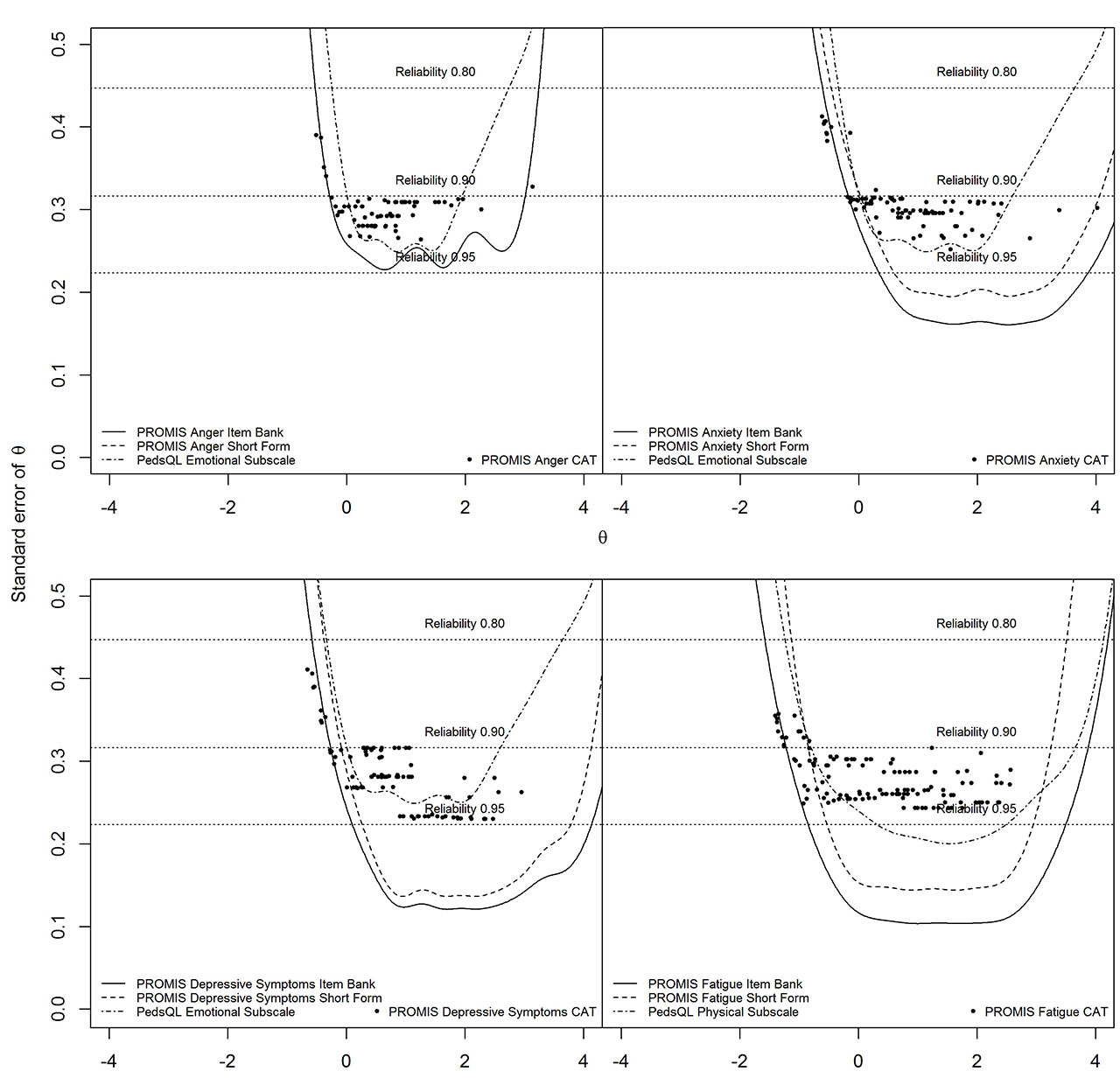
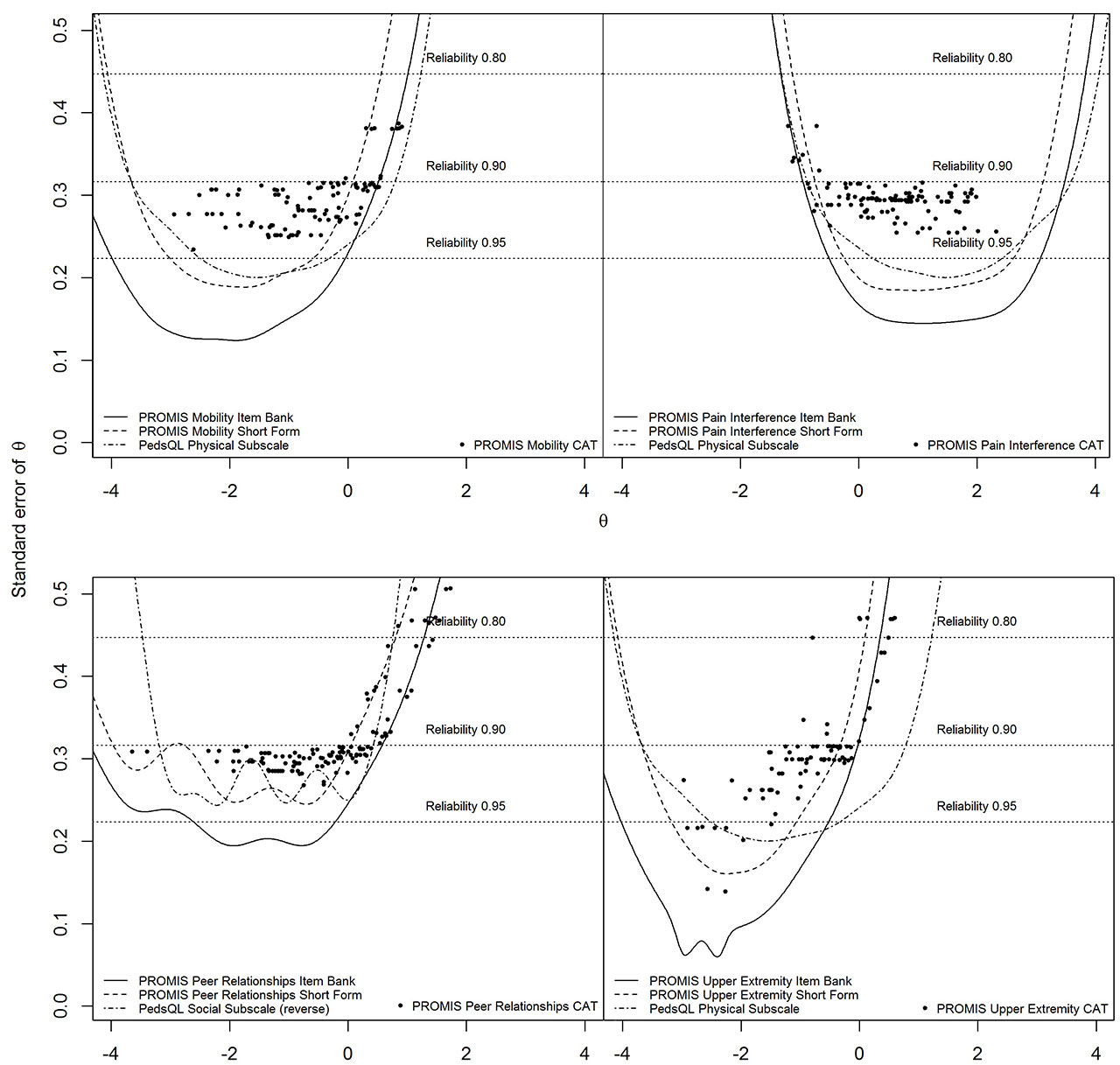
Methods: 154 Dutch children (8-18 years, mean=14.4, SD=3.0) with JIA completed eight pediatric v1.0 PROMIS item banks (Anger, Anxiety, Depressive Symptoms, Fatigue, Pain Interference, Peer Relationships, Physical Function Mobility, Physical Function Upper Extremity) twice and the Pediatric Quality of Life Inventory (PedsQL) and the Childhood Health Assessment Questionnaire (CHAQ) once. Structural validity of the item banks was assessed by fitting a Graded Response Model (GRM) and inspecting GRM fit (CLI, TLI, RMSEA) and item fit (S-X2). Convergent validity (with PedsQL/CHAQ subdomains) and discriminative validity (active/inactive disease) were assessed. Reliability of the item banks, short-forms and CATs was expressed as standard error of theta (SE(θ)). Test-retest reliability was assessed using intraclass correlation coefficients (ICC) and Smallest Detectable Change (SDC).
Results: All item banks had sufficient overall GRM fit (CFI >.95, TLI >.95, RMSEA< .08) and no item misfit (all S-X2 p >.001). High correlations ( >.70) were found between most PROMIS T-scores and hypothesized PedsQL/CHAQ (sub)domains. Mobility, Pain Interference and Upper Extremity item banks were able to discriminate between patients with active and inactive disease. Regarding reliability PROMIS item banks outperformed legacy instruments. Post-hoc CAT simulations outperformed short-forms. Test-retest reliability was strong (ICC >.70) for all full-length item banks and short-forms, except for Peer Relationships.
Conclusion: The pediatric PROMIS item banks displayed sufficient psychometric properties in children with JIA. CATs outperformed short-forms in terms of test length and amount of patients reliably estimated. PROMIS item banks are ready for use in clinical research and practice in children with JIA.
To cite this abstract in AMA style:
Luijten M, Terwee C, van Oers H, Joosten M, van den Berg M, Schonenberg-Meinema D, Dolman K, Ten Cate R, Roorda L, Grootenhuis M, van Rossum M, Haverman L. Psychometric Properties of the Pediatric Patient-Reported Outcomes Measurement Information System (PROMIS®) Item Banks in a Dutch Clinical Sample of Children with Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/psychometric-properties-of-the-pediatric-patient-reported-outcomes-measurement-information-system-promis-item-banks-in-a-dutch-clinical-sample-of-children-with-juvenile-idiopathic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/psychometric-properties-of-the-pediatric-patient-reported-outcomes-measurement-information-system-promis-item-banks-in-a-dutch-clinical-sample-of-children-with-juvenile-idiopathic-arthritis/