Session Information
Date: Sunday, October 21, 2018
Title: 3S111 ACR Abstract: Spondyloarthritis Incl PsA–Clinical II: PsA Epidemiology (964–969)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The GRAPPA-OMERACT PsA working group (WG) is using the OMERACT Filter 2.1 instrument selection algorithm1 to develop a Psoriatic Arthritis (PsA) core instrument set for randomized controlled trials (RCT) and longitudinal observational studies (LOS). The PsA Impact of Disease Instrument (PsAID12)2 is a candidate instrument for PsA-specific Health Related Quality of Life (HRQoL)3. Based on evidence and consensus, the WG formulated a recommendation for PsAID12 and aimed to obtain endorsement by OMERACT.
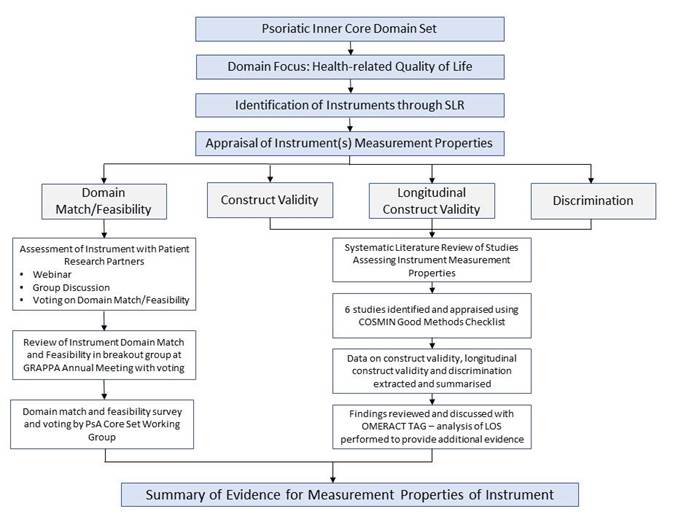
Methods: We used a multi-step process (Fig1). PSAID12 psychometric evidence to fulfill Filter 2.1 was gathered in a systematic literature review (SLR) of patient reported outcomes (PROM) in PsA3, and additional analyses conducted in an LOS. Analyses that not been published were independently reviewed by the OMERACT technical advisory group. Data were presented to stakeholders [WG, patient research partners (PRP), workshop at the GRAPPA 2017 annual meeting4] followed by a survey to vote on domain match and feasibility. All data were summarized as PsAID12 OMERACT pre-conference reading material. Data and process were presented, discussed in 8 breakout groups, and voted on, at the OMERACT2018 conference (Terrigal, Australia, May 2018).
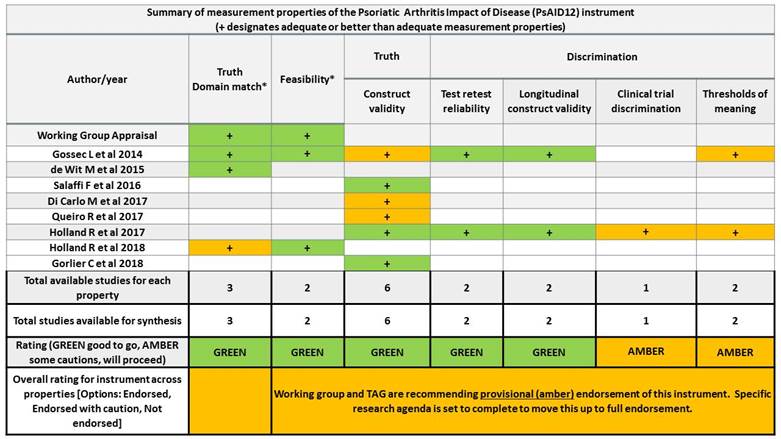
Results: An excerpt of the PsAID12 evidence presented at OMERACT 2018 is represented in Fig2. PsAID12 fulfilled with green (good to go) domain match, feasibility, reliability, and construct/longitudinal construct validity. Discrimination was assessed in an LOS within change groups. Minimal clinically important improvement in 2 LOS2,5 were 3 and 1.4. PsAID12 fulfilled with amber (provisional endorsement) discrimination and thresholds of meaning. The overall WG recommendation was formulated: amber/provisional endorsement of PsAID12 for measuring PsA specific-HRQoL in RCTs and LOS. Of 113 participants at the PsA OMERACT workshop 87% (97) voted “yes” endorsing this recommendation. 17 were PRPs and 93% voted “yes”. The WG set a research agenda to fully endorse PsAID12.
Conclusion: At OMERACT 2018, PsAID12 was the first PROM provisionally endorsed as core instrument to measure PsA-specific HRQoL in RCTs. PsAID12 discrimination and improvement thresholds will be studied in RCTs.
Refs: 1OMERACT handbook, 2Gossec 2014, 3Højgaard 2017, 4Holland 2018, 5 Holland 2017
 |
To cite this abstract in AMA style:
Holland R, Christensen R, Goel N, Hoejgaard P, Tillett W, Gossec L, de Wit M, McHugh N, Mease PJ, Shea B, Leung YY, Gladman DD, Lindsay C, Coates LC, Ogdie A, Strand V, Fallon L, Birt J, Callis Duffin K, FitzGerald O, Tugwell P, Beaton D, Orbai AM. Psoriatic Arthritis Impact of Disease (PsAID12) Was Provisionally Endorsed at Omeract 2018 As Core Instrument to Measure Psoriatic Arthritis-Specific Health-Related Quality of Life in Randomized Controlled Trials and Longitudinal Observational Studies [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/psoriatic-arthritis-impact-of-disease-psaid12-was-provisionally-endorsed-at-omeract-2018-as-core-instrument-to-measure-psoriatic-arthritis-specific-health-related-quality-of-life-in-randomized-contr/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/psoriatic-arthritis-impact-of-disease-psaid12-was-provisionally-endorsed-at-omeract-2018-as-core-instrument-to-measure-psoriatic-arthritis-specific-health-related-quality-of-life-in-randomized-contr/