Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Skin and joint symptoms both contribute to the burden of disease in psoriatic arthritis (PsA).1 More severe skin symptoms in patients with both skin and joint involvement have been reported to be associated with poorer quality of life, higher disability and lower work productivity.2 The objective of this study was hence to evaluate to what extent baseline (BL) factors, such as comorbid PsA and psoriasis (PsO) severity, are associated with ustekinumab (UST) persistence in patients with PsO enrolled in the British Association of Dermatologists Biologic and Immunomodulators Register (BADBIR).
Methods: This retrospective observational study used data from patients with PsO receiving UST in the BADBIR (first enrolled, 07/2009; data cut-off, 10/2020). Time to discontinuation (TTD) was defined as the time from treatment start date until treatment stop date, censoring for loss to follow-up where patients were lost to follow-up or active in the registry without a recorded treatment stop date. Hazard ratios (HRs), confidence intervals and p-values (Wald test) for the association between obesity, sex, PsA status, prior biologic exposure, depression, Psoriasis Area and Severity Index (PASI) strata, and interactions between covariates and TTD were generated using Cox proportional hazards regression. Kaplan–Meier analysis was used to estimate probability of TTD. Patients were stratified by PsA diagnosis at BL and PASI. The log-rank test was used to compare persistence among groups.
Results: Patient BL characteristics are listed in Table 1. Multivariable analysis (Table 2) found that median TTD was shorter for patients with comorbid PsA vs. PsO alone (HR 1.98; p< 0.0001). Median TTD was shorter in patients with depression vs. no depression (HR 1.21; p=0.0007). Men were at lower risk of discontinuation vs. women (HR 0.72; p< 0.0001). Prior biologic experience reduced TTD in patients with comorbid PsA vs. biologic-naïve patients with PsA (HR 2.35; p=0.005). PASI >3 at BL was associated with increased probability of discontinuation vs. PASI ≤3 (PASI >3–≤10, HR 1.35, p=0.002; PASI >10–≤20, HR 1.23, p=0.019; PASI >20, HR 1.77, p< 0.0001). In patients with comorbid PsA, higher skin severity at BL also led to a shorter TTD, with PsA seeming to have a greater impact on UST persistence in patients with lower skin severity at BL. Median TTD in patients with PsO alone and PASI ≤3 was 8.11 years vs. 5.27 years for patients with comorbid PsA (p< 0.0001). In patients with PASI >20, median TTD for patients with PsO alone was 5.33 years vs. 3.89 years for patients with comorbid PsA (p=0.018; Figure 1).
Conclusion: PsA, female sex and depression were associated with a shorter TTD of UST. More severe skin disease at BL had a higher association with discontinuation, irrespective of comorbid PsA, and comorbid PsA had a greater association with shorter TTD in patients with lower PASI scores. This suggests that patient-centric, multidisciplinary care needs to be considered to achieve the best possible outcomes in psoriatic disease.
References: 1. Merola et al. Rheumatol Ther 2019;6:33–45; 2. Tillett et al. Rheumatol Ther 2020;7:617–637.
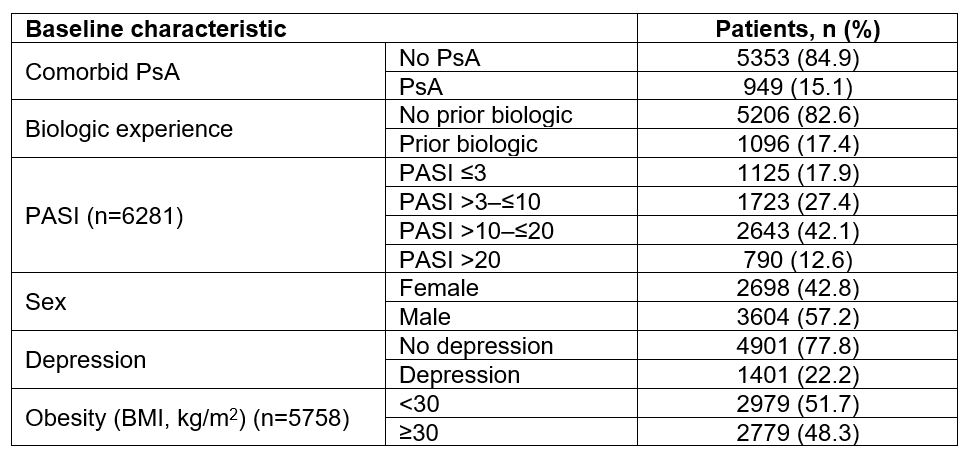 Table 1: Baseline characteristics of patients with PsO treated with UST in the BADBIR (Nf6302). BADBIR, British Association of Dermatologists Biologic and Immunomodulators Register; BMI, body mass index; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; UST, ustekinumab.
Table 1: Baseline characteristics of patients with PsO treated with UST in the BADBIR (Nf6302). BADBIR, British Association of Dermatologists Biologic and Immunomodulators Register; BMI, body mass index; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; UST, ustekinumab.
 Table 2: Cox regression model for time to discontinuation of UST in patients with PsO. *Indicates interaction between covariates. BMI, body mass index; HR, hazard ratio; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; UST, ustekinumab.
Table 2: Cox regression model for time to discontinuation of UST in patients with PsO. *Indicates interaction between covariates. BMI, body mass index; HR, hazard ratio; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; UST, ustekinumab.
PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; PsO, psoriasis; UST, ustekinumab.
To cite this abstract in AMA style:
Ogdie A, Tillett W, Passey A, Gorecki P. Psoriatic Arthritis, Female Sex and Increased Baseline Skin Severity Are Associated with Drug Persistence in Ustekinumab-Treated Patients with Psoriasis in the BADBIR Cohort [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/psoriatic-arthritis-female-sex-and-increased-baseline-skin-severity-are-associated-with-drug-persistence-in-ustekinumab-treated-patients-with-psoriasis-in-the-badbir-cohort/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/psoriatic-arthritis-female-sex-and-increased-baseline-skin-severity-are-associated-with-drug-persistence-in-ustekinumab-treated-patients-with-psoriasis-in-the-badbir-cohort/

