Session Information
Date: Monday, October 22, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster II: Clinical/Epidemiology Studies
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: We examined clinical correlations between disease activity, as measured with the Clinical Disease Activity for PsA (cDAPSA) components and PsA life impact/health-related quality of life (HRQoL) assessed with the PsA Impact of Disease Questionnaire (PsAID12). We hypothesized that PsAID12 scores will be statistically different among the disease activity categories and there will be moderate correlation between PsAID12 and cDAPSA.
Methods: LAPIS-PsA is a multicenter, prospective, non-interventional study assessing long-term apremilast (APR) treatment in adult patients with active PsA in routine clinical practice in Germany. Analyses were performed in patients treated with APR 30 mg BID who had cDAPSA and PsAID12 scores at baseline (BL). We also report data from Visits 1 to 3 (V1: ≥1 month; V2: ≥4 months; V3: ≥7 months). Mean PsAID12 scores and proportions of patients meeting the PsAID12 Patient-Acceptable Symptom State (PASS), defined as PsAID12 of 4, were assessed among each cDAPSA category at BL. PsAID12 scores were compared among the cDAPSA categories at BL and each visit using the ANOVA method. Pearson correlations were calculated between cDAPSA and PsAID12 at BL, V1, V2, and V3.
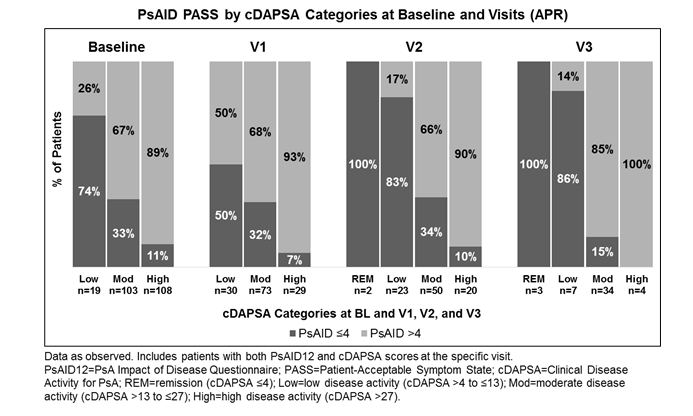
Results: A total of 330 patients were included in the analysis population. At BL, patients had a mean PsAID12 of 5.4 and a mean cDAPSA of 28.6. Mean PsAID12 and cDAPSA scores are reported at all visits (Table). Patients reported incrementally reduced mean cDAPSA and consistent improvements in PsAID12 scores from BL to V3. Patients with low disease activity (cDAPSA >4−≤13) at BL had a mean (SD) PsAID12 score of 3.2 (1.5) with 74% in PsAID12 PASS (Figure). Those with moderate (cDAPSA >13−≤27) and high (cDAPSA >27) disease activity had mean (SD) PsAID12 scores of 5.0 (2.0) and 6.1 (1.7), with 33% and 11% showing PsAID12 PASS, respectively. Consequently, proportions of patients achieving PsAID12 PASS decreased with higher disease activity (Figure). At BL and V1-3, PsAID12 scores were significantly different among the cDAPSA categories (P<0.001). Additionally, significant moderate to high correlations were observed between cDAPSA and PsAID12 at V1 (Pearson’s coefficient r=0.41; P<0.0001), V2 (r=0.64; P<0.0001), and V3 (r=0.70; P<0.0001).
Conclusion: Results from this observational PsA study of APR demonstrate a significant correlation between disease activity, as assessed by cDAPSA, and PsA-specific life impact/HRQoL, as measured by PsAID12. More patients met the PsAID12 PASS cutoff at lower disease activity levels, suggesting that improvements in PsA disease activity lead to decreased impact of disease.
To cite this abstract in AMA style:
Orbai AM, Krüger K, Behrens F, Kiltz U, Guerette B, Mellars L, Brunori M, Wollenhaupt J. Psa Impact of Disease Questionnaire Scores Are Correlated with Disease Activity, As Measured By Cdapsa in Patients with Psa [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/psa-impact-of-disease-questionnaire-scores-are-correlated-with-disease-activity-as-measured-by-cdapsa-in-patients-with-psa/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/psa-impact-of-disease-questionnaire-scores-are-correlated-with-disease-activity-as-measured-by-cdapsa-in-patients-with-psa/