Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Drug induced subacute cutaneous lupus erythematosus has rarely been described. There is a growing literature reporting the association between proton pump inhibitor (PPI) use and subacute cutaneous lupus erythematosus (SCLE).
To compare the clinical characteristics of a cohort of patients with proton pump inhibitor induced subacute cutaneous lupus erythematosus, their clinical course and treatment with a control group of primary subacute cutaneous lupus erythematosus patients not exposed to proton pump inhibitors.
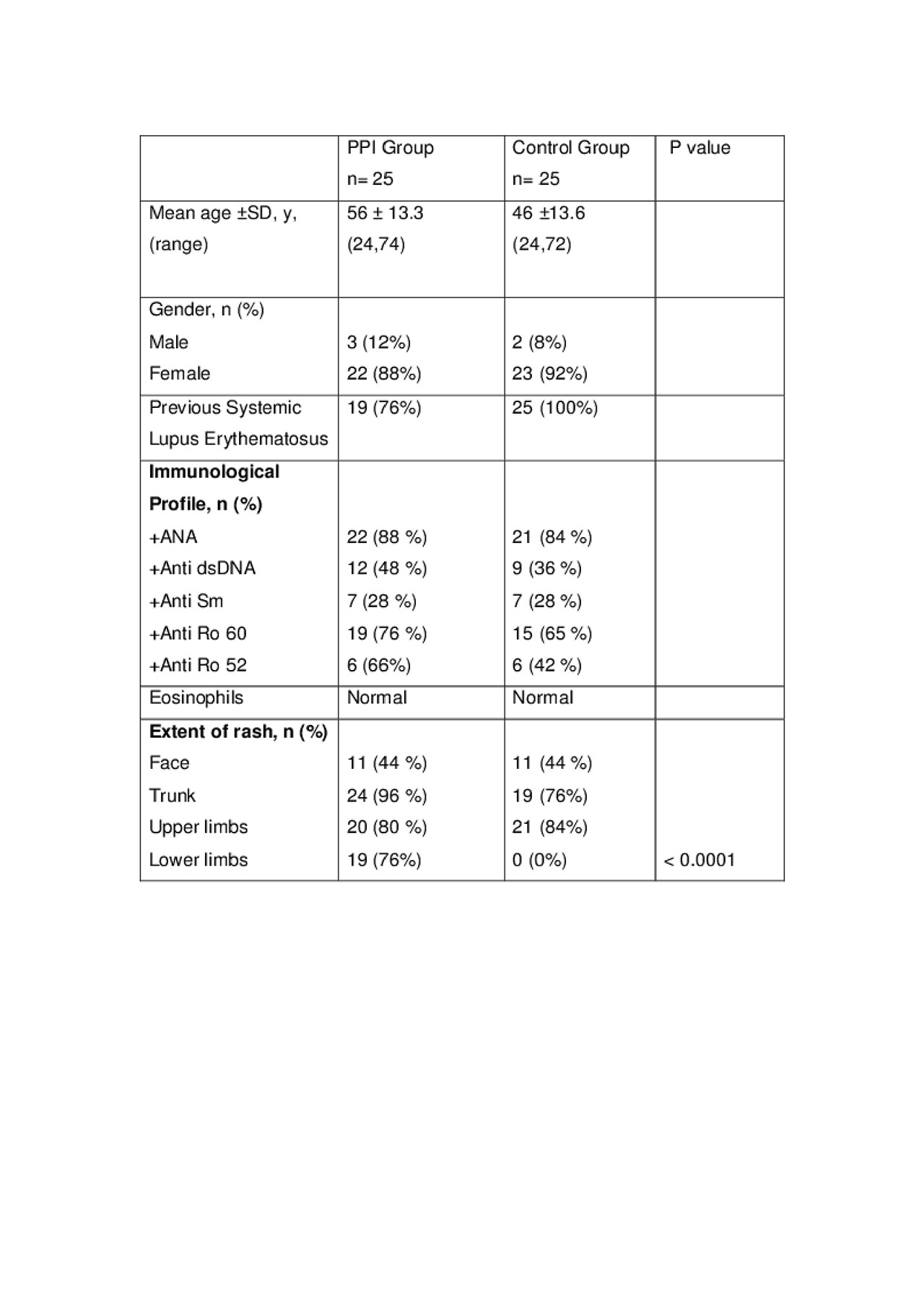
Methods: A matched case-control study in a tertiary referral setting at the Louise Coote Lupus Unit. There were 50 SCLE patients (25 patients with PPI induced SCLE and 25 patients with Primary SCLE). Clinical details recorded included underlying systemic lupus erythematosus (SLE) and SCLE, extent of cutaneous involvement, time to presentation, type of proton pump inhibitor and dosage, histological characteristics, immunological profiles, treatment and time to resolution.
Results: There were 25 patients in the PPI group and 25 patients in the Primary SCLE group. There was no significant gender difference, the mean age at diagnosis was 56.3 years for PPI group and 46 years for Primary SCLE group. Nineteen patients (76%) had underlying SLE in the PPI group. In this subgroup, only one patient had SCLE as part of their initial SLE presentation. Lower limb skin lesions were significantly more prevalent in the PPI group (p< 0.0001). No significant immunological profile difference was observed between the two groups in terms of anti-double stand DNA, anti Ro-60 antibody and Anti-Sm antibodies. The prevalence of anti Ro-52 antibodies was slightly higher in PPI group 66% compared with 42% in Primary SCLE group. Peripheral blood eosinophils were normal in all patients in the PPI group, supporting the hypothesis of drug induced SCLE rather than a true allergic reaction. Twelve patients underwent a skin biopsy in the PPI group and 11 patients had histology in keeping with SCLE. The median time to presentation was 12 months (range 3 weeks – 12 years) and median resolution period was 1 month (range 1 week- 5 months). Proton pump inhibitor was stopped in 23 patients while 2 patients could not stop it for another clinical indications. Ten patients (40%) received concurrent oral corticosteroid.
Conclusion: Proton pump inhibitor induced SCLE is more common in older patients and may be associated with anti-Ro 52 antibodies. Lower limb involvement is a pointer to PPI induced SCLE. Clinical resolution of the rash may take few days up to a few months. This is likely a class effect with all proton pump inhibitors.
To cite this abstract in AMA style:
Alrashid A, Poh Y, D'Cruz D, Sangle S, McGibbon D, Benton E, Higgins E. Proton Pump Inhibitor Induced Subacute Cutaneous Lupus Erythematosus: Clinical Characteristics and Outcomes – Case Control Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/proton-pump-inhibitor-induced-subacute-cutaneous-lupus-erythematosus-clinical-characteristics-and-outcomes-case-control-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proton-pump-inhibitor-induced-subacute-cutaneous-lupus-erythematosus-clinical-characteristics-and-outcomes-case-control-study/