Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Tildrakizumab (TIL) is a high-affinity anti–interleukin-23p19 monoclonal antibody approved in the US, EU, and Australia to treat moderate to severe plaque psoriasis.1 A randomized, double-blind, placebo-controlled, multiple-dose, phase 2b study evaluating the efficacy and safety of TIL in psoriatic arthritis (PsA) was recently completed (NCT02980692). The objective of this analysis was to characterize and evaluate the rate of minimal disease activity (MDA) up to week (W)52 from the phase 2b study.
Methods: Patients (pts) ≥18 years old with active PsA2 and ≥3 tender and ≥3 swollen joints were randomized 1:1:1:1:1 to receive TIL 200 mg every 4 weeks (Q4W) to W52, TIL 200 mg every 12 weeks (Q12W) to W52, TIL 100 mg Q12W to W52, TIL 20 mg Q12W to W24→TIL 200 mg Q12W to W52, or placebo (PBO) Q4W to W24→TIL 200 mg Q12W to W52. MDA was assessed throughout the study; an MDA response was achieved when 5 of 7 criteria were met.3 Safety was assessed throughout the study and included treatment-emergent adverse event (TEAE) monitoring.
Results: Of 500 pts screened, 391 were randomized and received ≥1 dose of study drug. At baseline, mean age was 48.8 years, 55% were female, 97% were White, mean body mass index was 29.7 kg/m2, and pts had PsA for a median (range) of 4.4 (0–42.8) years since diagnosis. Baseline disease characteristics related to MDA varied little between study arms (Table 1).
Significantly more patients receiving TIL vs PBO achieved MDA (19%–34% vs 6%; P ≤0.0172) by W24; the proportion further increased with continued TIL treatment to W52 (35%–48%), including pts who switched from PBO to TIL (37%) (Figure). At W24, there was a greater proportion of responders in TIL 200 mg Q12W and Q4W arms vs PBO across all MDA subcomponents except for tender entheseal points ≤1 and Health Assessment Questionnaire-Disability Index ≤0.05 (Table 2).
Among the overall pt population 64.5% and 3.3% experienced a TEAE and serious TEAE, respectively. Two (0.5%) pts had a fungal skin infection (candida, TIL 200 mg Q4W arm). One (0.3%) malignancy (intraductal proliferative breast lesion) occurred in the TIL 20→200 mg Q12W arm. One serious infection (chronic tonsillitis) was reported in the TIL 20 mg Q12W arm during the first 24 weeks. There were no reports of systemic candidiasis, uveitis, inflammatory bowel disease, major adverse cardiac events, or deaths over the 52 weeks of the study.
Conclusion: TIL produced clinically meaningful improvement in pts with PsA, resulting in a large proportion of pts achieving MDA by W52, and was well tolerated through W52. A 2-trial phase 3 program is underway.
References:
- Reich, et al. Lancet 2017;390:276−88.
- Taylor, et al. Arthritis Rheum 2006;54:2665–73.
- Coates, et al. Ann Rheum Dis 2010;69:48−53.
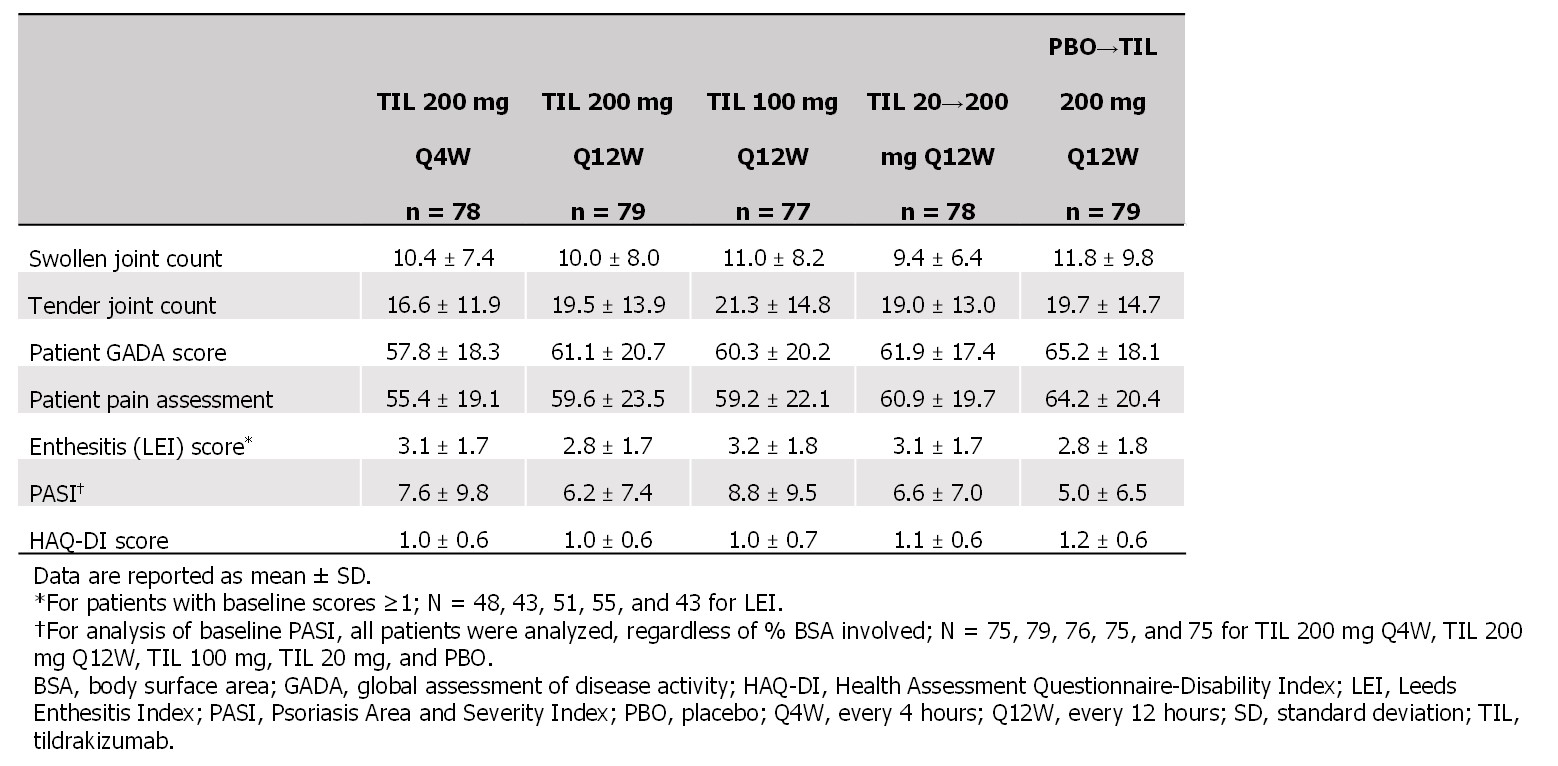 Table 1. Baseline disease characteristics related to minimal disease activity
Table 1. Baseline disease characteristics related to minimal disease activity
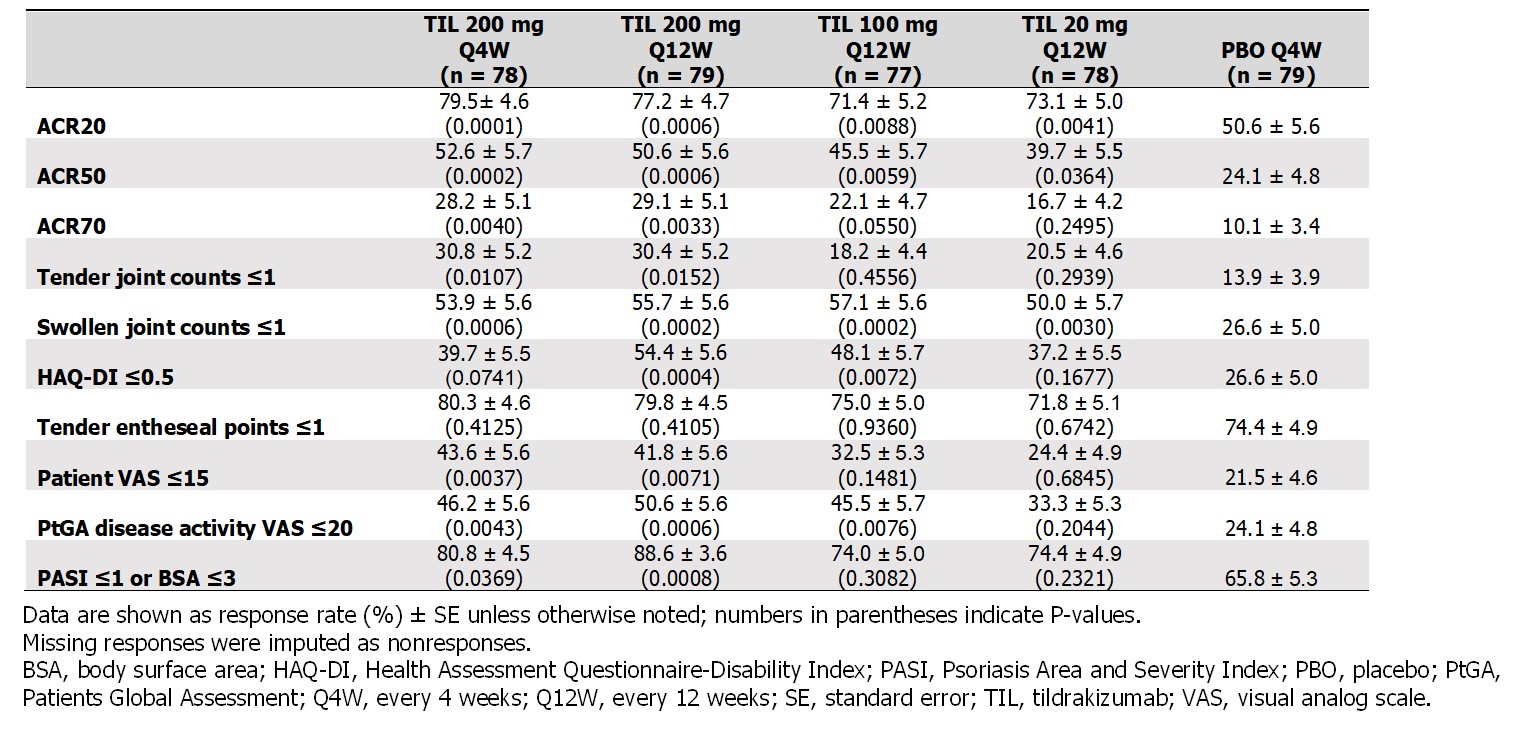 Table 2. Efficacy outcomes related to minimal disease activity at week 24
Table 2. Efficacy outcomes related to minimal disease activity at week 24
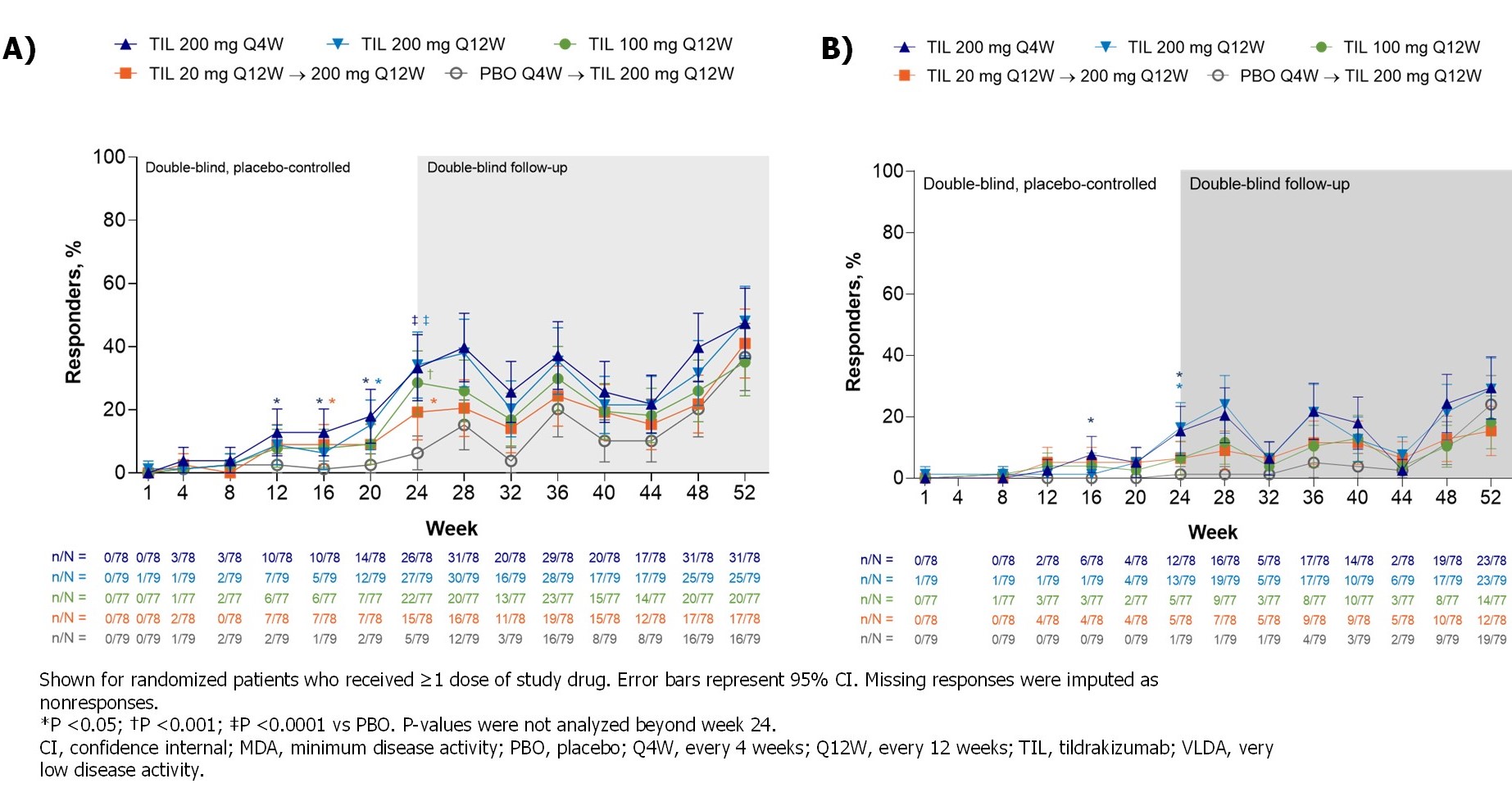 Figure. MDA responders (A) over time and (B) VLDA responders by treatment and time point
Figure. MDA responders (A) over time and (B) VLDA responders by treatment and time point
To cite this abstract in AMA style:
Nash P, Luggen M, García Fructuoso F, Chou R, Mendelsohn A, Rozzo S, McInnes I. Proportions of Patients Achieving a Minimal Disease Activity State upon Treatment with Tildrakizumab in a Psoriatic Arthritis Phase 2b Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/proportions-of-patients-achieving-a-minimal-disease-activity-state-upon-treatment-with-tildrakizumab-in-a-psoriatic-arthritis-phase-2b-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/proportions-of-patients-achieving-a-minimal-disease-activity-state-upon-treatment-with-tildrakizumab-in-a-psoriatic-arthritis-phase-2b-study/
