Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Medication, procedure, and diagnosis coding using structured data is fundamental to using real-world data to support clinical research, impacting cohort selection, patient recruitment, and outcome measurement. Source vocabulary systems (e.g. ICD-10-CM, HCPCS, RxNorm) are updated regularly, and incorporating these changes into existing code sets is essential yet challenging to manage, particularly in multidisciplinary settings. To address this need, we developed CodeMapper, an informatics tool designed to facilitate routine code updates and interdisciplinary collaboration. Our objective was to quantify medication, procedure, and diagnosis changes over time to assess the importance of maintaining updated code sets and provide a foundation for tool development.
Methods: We analyzed codes using the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes from the National Center for Health Statistics and National Drug Codes (NDC) from First Data Bank (2015 to 2024). We also assessed Healthcare Common Procedure Coding System (HCPCS) codes from Centers for Medicare & Medicaid Services from inception through 2024. We summarized code changes over time and visualized trends using figures.
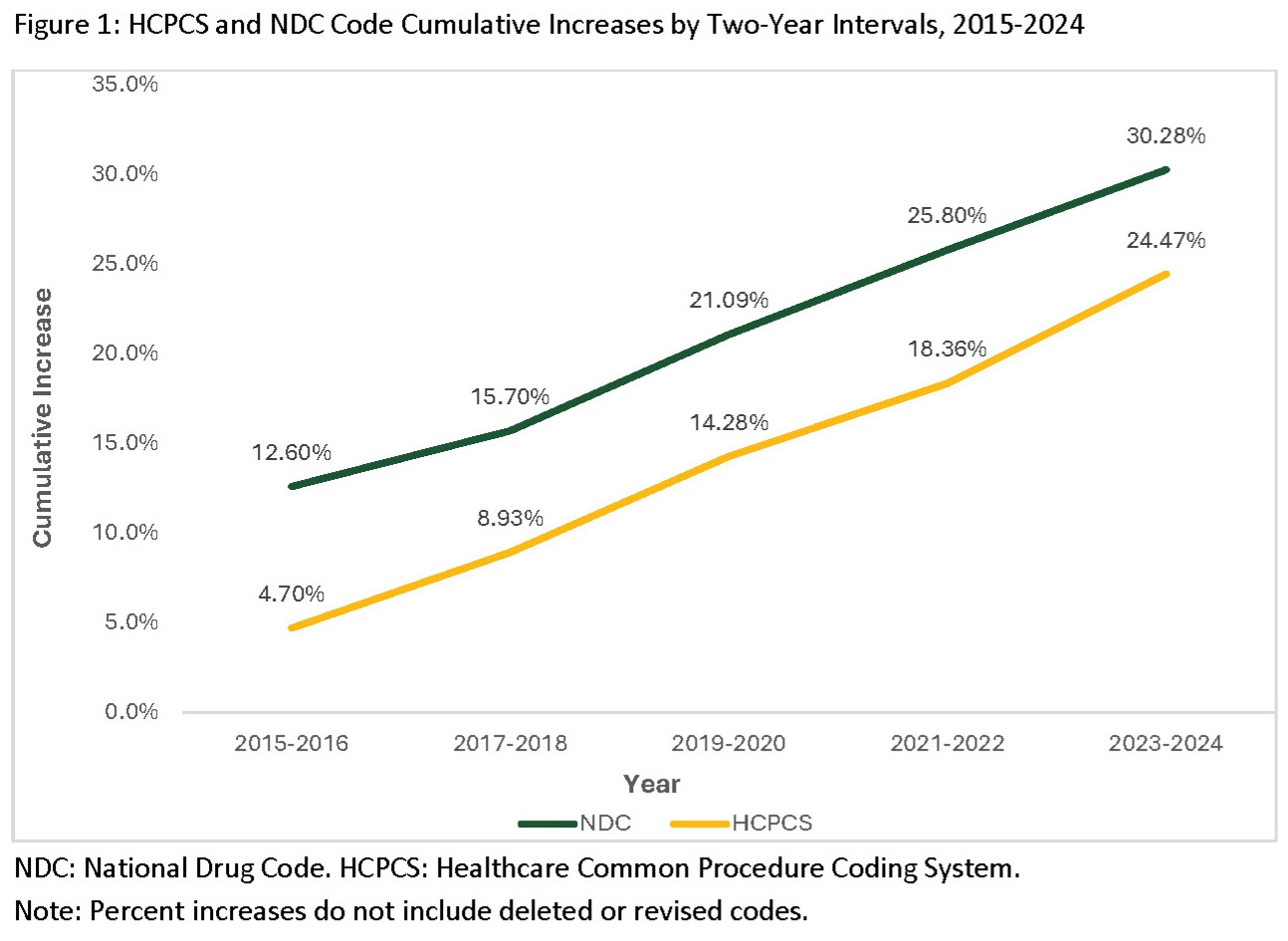
Results: Between 2015 to 2024, 2,911 new HCPCS codes were added. These additions accounted for approximately 25% of all HCPCS codes (Figure 1). Similarly, notable increases in the number of NDC codes occurred from 2016-2024 with a median (IQR) of 20510.0 (20424.0, 22275.0) NDC codes added per two-year period (Figure 1). Similar changes were also observed for ICD-10-CM codes with a median of 377.5 (276.0, 833.0) additions, 54.5 (30.0, 214.5) deletions, and 38.5 (24.0, 184.5) revisions occurring annually (Figure 2). Revisions describe the changes occurring in naming, coding granularity, and numbering. Given these substantial changes over time in standardized coding systems, we developed CodeMapper, a new web-based informatics tool integrating current and historical diagnostic, procedural, medication, and laboratory codes. With semi-annual source vocabulary updates, this tool helps maintain the code sets described above and offers the ability for interdisciplinary teams to collaborate and refine code lists for use in current or future research (Figure 3).
Conclusion: Regular updates to medical coding systems are essential to support accurate research findings. The potential to under-ascertain codes within structured data is high, particularly in multi-year longitudinal studies where code sets may be fixed at the time of protocol development yet will under-capture medications, procedures, and diagnoses as they change over time. Maintenance and integration of current and complete source vocabularies are critical for rigorous and generalizable clinical research both for trials and real-world evidence using observational electronic health record or claims data.
To cite this abstract in AMA style:
Holladay E, McCormick N, Matthews R, Daigle S, Xie F, Mehta T, Curtis J. Promoting Health Services Research Rigor and Reproducibility using CodeMapper: A novel tool for harmonization, collaboration, and precision [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/promoting-health-services-research-rigor-and-reproducibility-using-codemapper-a-novel-tool-for-harmonization-collaboration-and-precision/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/promoting-health-services-research-rigor-and-reproducibility-using-codemapper-a-novel-tool-for-harmonization-collaboration-and-precision/

.jpg)
.jpg)