Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) symptoms and quality of life are core domains for PsA assessment1. The PROMIS Profile29 measures symptoms and quality of life using a T-score metric referenced to the general population, and has not been used in PsA before. The objective was to assess construct validity of the PROMIS Profile29 in PsA.
Methods: Participants with PsA were followed every 3-6 months in conjunction with rheumatology clinic visits. The PROMIS Profile29 was collected concomitantly with PsA specific measures: clinical Disease Activity Score in Psoriatic Arthritis (cDAPSA), Minimal Disease Activity (MDA), and the Psoriatic Arthritis Impact of Disease (PsAID)2. Multiple anchors were used to define treat-to-target (T2T) status: MDA vs not MDA3, cDAPSA T2T (cDAPSA< 14) vs not3, and the PsAID patient acceptable symptoms state (PsAID score< 4)2. We hypothesized mean PROMIS scores will be worse in patients not at T2T vs T2T, and that correlations with PsAID and cDAPSA will be moderate-high and low-moderate, respectively. We hypothesized lower correlations between change scores versus point scores due to measurement error.
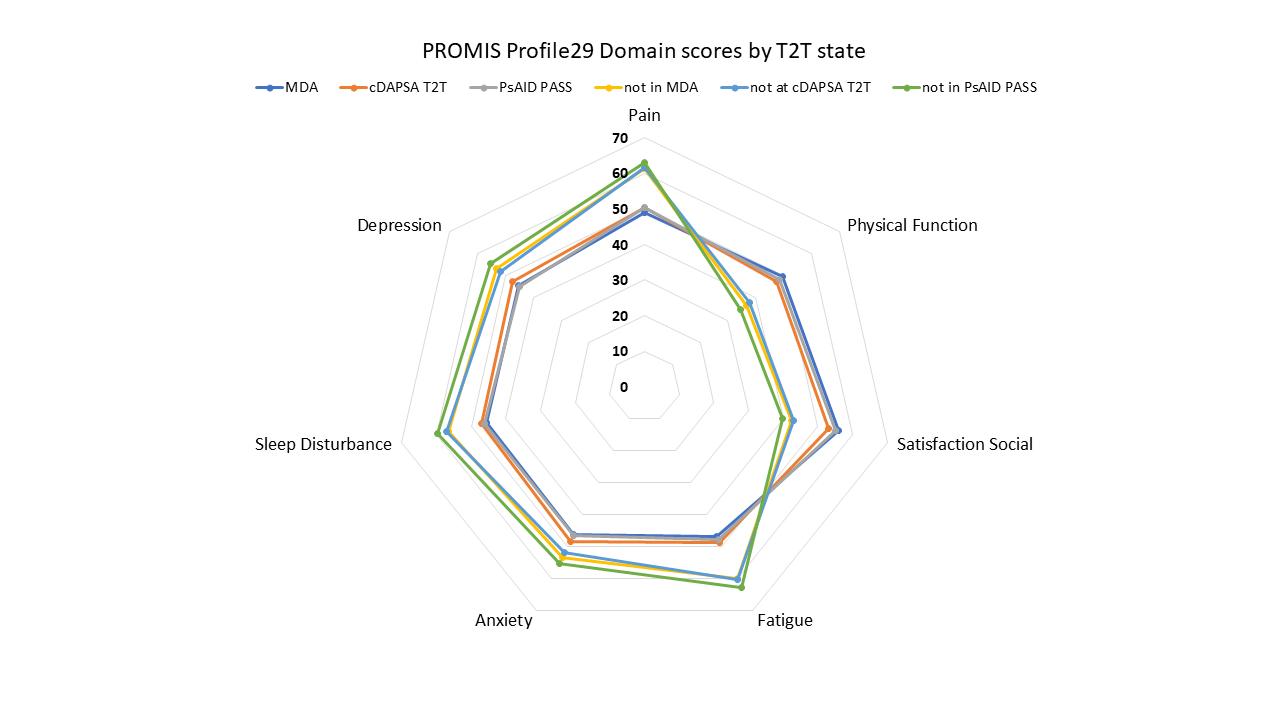
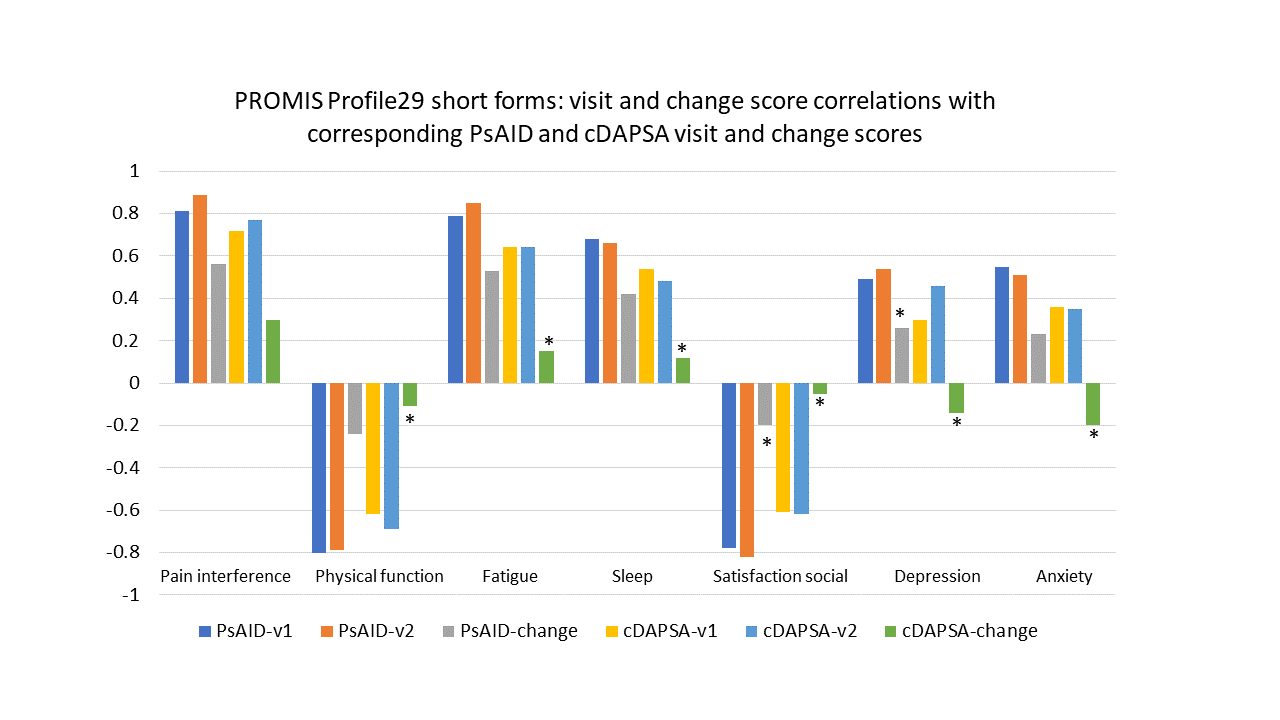
Results: One hundred participants had a baseline visit and 93 a second study visit, all met CASPAR PsA classification criteria. Mean age (SD) was 52 (12) years, PsA disease duration 17.7 (13) years, 88% were white and 60% female. At baseline, mean (SD) tender (out of 68)/swollen (out of 66) joints were 3.2 (4.9)/3.1 (3.7), 8% had enthesitis, 3% dactylitis; mean(SD) HAQ-DI 0.7(0.8), PsAID 3.21 (2.4), pain VAS 36 (28.7)mm, patient global PsA 37.7 (31.7)mm. MDA state was met by 50% and DAPSA treat to target (T2T) by 55%. Treatment included biologicals alone/in DMARD combination in 55% and DMARD alone in 45%. PROMIS Profile29 T-scores (Figure 1) were significantly better across all domains for participants at T2T versus not, using MDA status and PsAID PASS. cDAPSA T2T status grouping yielded similar results, except for PROMIS Anxiety which was not significantly different. Results were consistent at both visits. Spearman correlations with PsAID were very high ( >0.8) for PROMIS Profile29 Pain interference, Physical function, Fatigue and Satisfaction with social roles, high ( >0.6) for Sleep, and moderate ( >0.4) for Depression and Anxiety. Correlations with cDAPSA were high for Pain, Physical function, Fatigue and Satisfaction, moderate with Sleep, and low with Depression and Anxiety. Change score correlations were in the expected direction and lower than point correlations between Profile29 forms and PsAID, except for Depression (not significant). Change score correlations with cDAPSA were low and significant for Pain interference but not the other domains.
Conclusion: PROMIS Profile29 differentiated PsA T2T state from active disease using multiple anchors. Point PROMIS Profile 29 T-scores had moderate-high correlation with PsAID scores; and change scores had low-moderate correlation with PsAID change scores, meeting pre-specified hypotheses. The PROMIS Profile29 can be used to assess clinical status in PsA and it aligns with PsA specific disease activity and life impact measures.
Reference: 1. Orbai A, et al. Ann Rheum Dis 2017; 2.Gossec L, et al. Ann Rheum Dis 2014; 3. Smolen J, et al. Ann Rheum Dis. 2018;
To cite this abstract in AMA style:
Orbai A, Manno R, Perin J, Kim N, Smith K, Wu A, Bingham C, Haque U. PROMIS Profile29 Differentiates Active Disease from Treat-to-Target State in Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/promis-profile29-differentiates-active-disease-from-treat-to-target-state-in-psoriatic-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/promis-profile29-differentiates-active-disease-from-treat-to-target-state-in-psoriatic-arthritis/