Session Information
Date: Tuesday, November 10, 2015
Title: 2015 Rheumatology Research Foundation Edmond L. Dubois, MD Memorial Lectureship
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Systemic
lupus erythematosus (SLE) is an autoimmune disease causing inflammation
throughout the body and cardiovascular involvement is a significant contributor
to morbidity and mortality in this patient population. We have previously identified data demonstrating
impaired left ventricular function using cardiac magnetic resonance
(CMR) with quantitative T2 mapping in patients with SLE during a flare, which is
suggestive of subclinical myocardial inflammation. With the early stages of cardiac
disease clinically silent, understanding the underlying pathogenic
contributions of autoimmune-mediated inflammation would enable more effective
therapeutic intervention. Thus, the objective of this study was to establish a
T2 mapping protocol in an animal model of lupus to examine cardiac function longitudinally
in order to better understand the pathobiology of SLE on cardiovascular
disease.
Methods: Kidney function was
assessed by weekly blood urea nitrogen (BUN) testing in NZM2410 mice, which develop
severe lupus-like glomerulonephritis at 22-40 weeks of age. Quantitative analysis of in vivo physiological cardiac function
was performed using a high-frequency
ultrasound system. Myocardial edema was quantitatively measured
in vivo with T2 CMR mapping on the BioSpec 94/30 microMRI imaging system. Heart tissue was collected for H&E and
Masson’s trichrome staining.
Results: For comparative
analysis in this study, wild-type mice were examined along with NZM2410 mice with
early-stage or late-stage lupus, as determined by weekly monitoring of BUN
levels. Echocardiograms revealed differences in cardiac output, diameter,
ejection fraction, fractional shortening, and stroke volume, which indicates
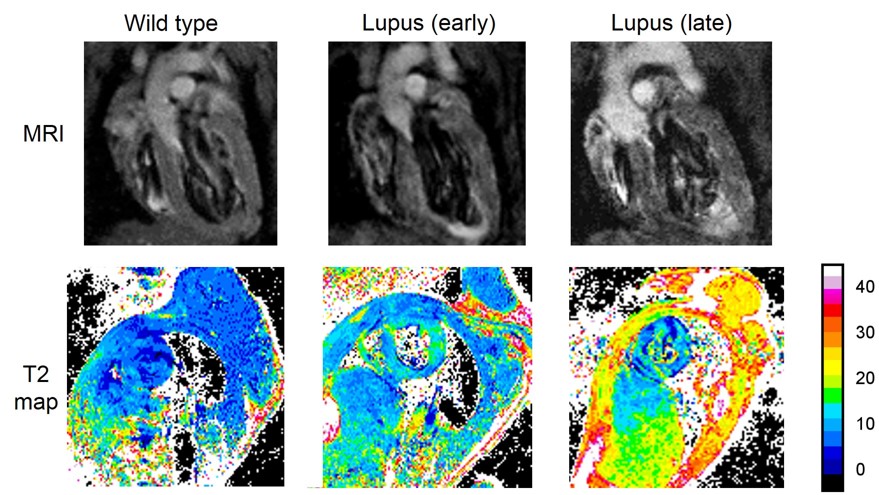
decreased cardiac function in late-stage NZM2410 mice. CMR analysis of sagittal
heart sections showed progressive thickening of the myocardium with a prominent
left ventricular hypertrophy. T2 CMR
mapping showed prolonged T2 signals, consistent with edema, in early and late
stage lupus hearts compared to wild type mice. To examine histopathology,
heart tissue was collected from early and late-stage NZM2410 mice for staining
with H&E and Masson’s trichrome. Evidence of myocarditis and fibrosis was
observed in mice with late-stage disease.
Conclusion: Our results
suggest an association of SLE disease progression with the development of cardiac
abnormalities resulting from inflammation in NZM2410 mice. Therefore, this
animal model could be used to further characterize the pathophysiological
correlation, relationship, and interplay between lupus and cardiovascular
disease in longitudinal studies. Future work will involve identification of
biomarkers related to cardiac involvement and will further develop CMR with
quantitative T2 mapping as a clinical tool to assess inflammation in patients
with SLE.
To cite this abstract in AMA style:
Young N, Hampton J, Aqel S, Pyles J, Bratasz A, Kalyanasundaram A, Jarjour WN, Ardoin SP. Progression of Lupus Pathology Is Correlative with Cardiac Magnetic Resonance Imaging Abnormalities, Diminished Function, and Inflammatory Histopathology in an Animal Model [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/progression-of-lupus-pathology-is-correlative-with-cardiac-magnetic-resonance-imaging-abnormalities-diminished-function-and-inflammatory-histopathology-in-an-animal-model/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/progression-of-lupus-pathology-is-correlative-with-cardiac-magnetic-resonance-imaging-abnormalities-diminished-function-and-inflammatory-histopathology-in-an-animal-model/