Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The ability to predict responses to apremilast (APR) could impact treatment decisions. This post-hoc analysis was conducted to (1) assess the predictive values of baseline (BL) clinical disease status on achieving long-term Clinical Disease Activity for Psoriatic Arthritis (cDAPSA) targets at Week 52 and (2) examine the association between early response to APR at Week 16 and the achievement of cDAPSA targets at Week 52.
Methods: Pooled analyses of 3 phase III trials (PALACE 1-3) were performed among subjects assigned to receive APR 30 mg BID at BL. Data were analyzed using multiple imputation to account for subjects who discontinued or had missing values, using all available cDAPSA scores. Probability of shifting across different cDAPSA categories from BL to Week 52 was calculated within these subjects. Binary logistic regression was also performed to confirm the results. To determine the extent of changes (from BL to Week 16) associated with achieving treatment targets at Week 52, we analyzed mean cDAPSA over time (from BL to Week 52) by cDAPSA category at Week 52.
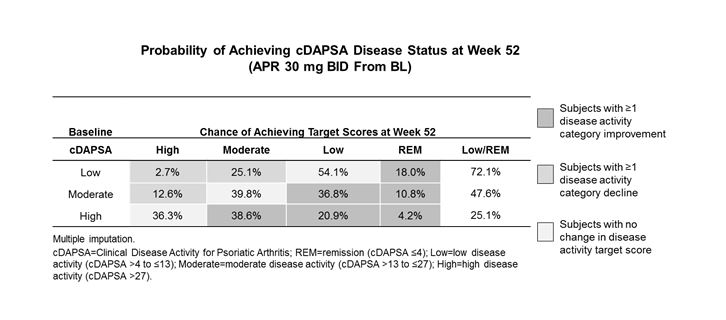
Results: A total of 496 subjects who received APR were included in the analyses. The estimated probabilities of achieving either cDAPSA low disease activity (LDA) or remission (REM) at Week 52 were 47.6% in subjects with BL moderate disease activity (cDAPSA >13 to ≤27) and 72.1% in subjects with BL LDA (cDAPSA >4 to ≤13) (Table). In subjects with BL high disease activity (cDAPSA >27), the probability of achieving LDA or REM by Week 52 was 25.1%. Binary logistic regression provided similar results. In subjects with moderate or low disease activity at BL, mean cDAPSA scores ranged from 21.0 to 22.4. Among these subjects, those who further achieved Week 52 LDA and REM had a mean cDAPSA at Week 16 of 14.9 and 8.2, respectively, suggesting that a reduction of ≥30% in cDAPSA by Week 16 predicts achievement of LDA or REM at Week 52.
Conclusion: Subjects with low or moderate disease activity at BL exhibited the highest likelihood of achieving and maintaining an improvement in LDA or REM with continued APR treatment to Week 52. Early response, based on changes in mean cDAPSA level from BL to Week 16, was associated with the achievement or maintenance of cDAPSA LDA or REM by Week 52.
To cite this abstract in AMA style:
Mease PJ, Behrens F, Gladman DD, Kavanaugh A, Brunori M, Teng L, Guerette B, Queiro R, Ogdie A. Probability of Achieving Low Disease Activity or Remission in Subjects with Active Psa Treated with Apremilast [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/probability-of-achieving-low-disease-activity-or-remission-in-subjects-with-active-psa-treated-with-apremilast/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/probability-of-achieving-low-disease-activity-or-remission-in-subjects-with-active-psa-treated-with-apremilast/