Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: During 16 weeks of blinded treatment, ixekizumab (IXE) and an adalimumab (ADA) active reference arm were found superior to placebo (PBO) in improving signs and symptoms of radiographic axial spondyloarthritis (r-axSpA).1 Here, we assess the safety and efficacy of continuous treatment with IXE through 52 weeks in patients with r-axSpA and describe clinical response at Week 52 for patients who switched to IXE following 16 weeks of treatment with either ADA or PBO.
Methods: Participants were biologic disease-modifying anti-rheumatic drug (bDMARD)-naïve adult patients with active r-axSpA per Assessment of Spondyloarthritis (SpA) international Society (ASAS) criteria (sacroiliitis centrally defined by modified New York criteria and ≥1 SpA feature) and inadequate response or intolerance to non-steroidal anti-inflammatory drugs. Patients were randomized 1:1:1:1 to receive 80 mg IXE every 2 (Q2W) or 4 weeks (Q4W), 40 mg adalimumab (ADA) Q2W (active reference arm), or PBO. At Week 16, patients assigned to IXE continued their assigned treatment and patients receiving PBO or ADA were re-randomized 1:1 to IXE Q2W or IXE Q4W through Week 52.
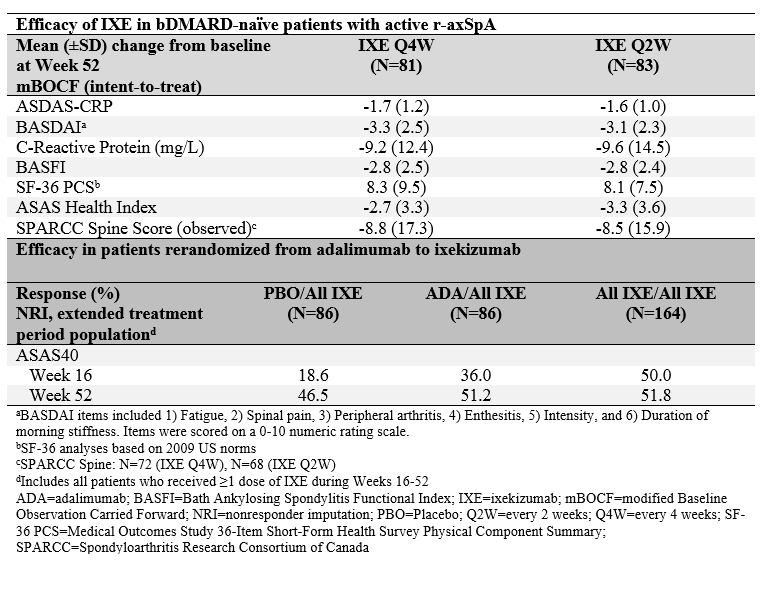
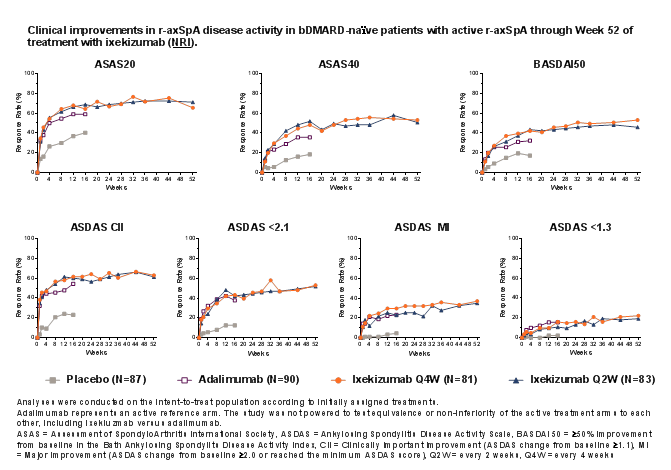
Results: Of 164 patients initially randomized to IXE, 146 (89%) completed Week 52. IXE Q4W and IXE Q2W led to persistent improvements in disease activity, function, objective inflammation (MRI and C-reactive protein), quality of life, health status, and overall functioning for up to 52 weeks (Figure and Table). For patients initially assigned to PBO or ADA, ASAS40 response showed a numerical increase upon switching to IXE (Table). Frequencies of treatment-emergent adverse events (AEs) were similar between IXE dosing regimens. Among patients with ≥1 dose of IXE (N=336), serious AEs occurred in 20 (6%) patients. There were no deaths and 11 (3%) patients discontinued due to AEs.
Conclusion: Persistent improvements in the signs and symptoms of r-axSpA were observed through Week 52 in patients who received continuous treatment with IXE. ASAS40 response rates at Week 52 were numerically similar between patients who received continuous treatment with IXE and patients who switched from ADA to IXE. No unexpected safety signals were observed through 52 weeks of treatment.
Reference: 1. van der Heijde et al. Lancet, 2018.
To cite this abstract in AMA style:
Wei C, Gensler L, Walsh J, Landewé R, Tomita T, Zhao F, Gallo G, Carlier H, Dougados M. Primary 1-Year Data of Ixekizumab in Biologic Disease-Modifying Anti-rheumatic Drug-Naïve Patients with Radiographic Axial Spondyloarthritis Including Data in Patients Rerandomized from Adalimumab to Ixekizumab [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/primary-1-year-data-of-ixekizumab-in-biologic-disease-modifying-anti-rheumatic-drug-naive-patients-with-radiographic-axial-spondyloarthritis-including-data-in-patients-rerandomized-from-adalimumab-to/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/primary-1-year-data-of-ixekizumab-in-biologic-disease-modifying-anti-rheumatic-drug-naive-patients-with-radiographic-axial-spondyloarthritis-including-data-in-patients-rerandomized-from-adalimumab-to/