Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is a heterogeneous, chronic inflammatory disease of the skin and musculoskeletal system. Six key domains of PsA have been identified to help guide treatment: peripheral arthritis, axial disease, enthesitis, dactylitis, nail psoriasis, and skin disease.1 Understanding the epidemiology of these different disease presentations is important for the management and treatment of PsA, yet there is limited evidence available. We aim to describe the prevalence of disease domain presentations among patients with PsA at enrollment in the Corrona PsA/SpA Registry.
Methods: This study included adult patients with PsA enrolled in the Corrona PsA/SpA Registry between March 2013 and August 2018. Patients were evaluated for the presence of 6 disease domains at enrollment: enthesitis (Spondyloarthritis Research Consortium of Canada enthesitis count > 0), dactylitis (dactylitis count > 0), peripheral arthritis (PA; tender and/or swollen joint count > 0), nail psoriasis (global nail psoriasis visual analog scale > 0), axial disease (physician-reported presence of spinal involvement, based on clinical judgment and/or radiographs or MRI showing sacroiliitis), and skin disease (BSA > 0%). The most common mutually exclusive disease presentations were summarized among all patients with PsA and those who initiated biologics using frequency counts and percentages.
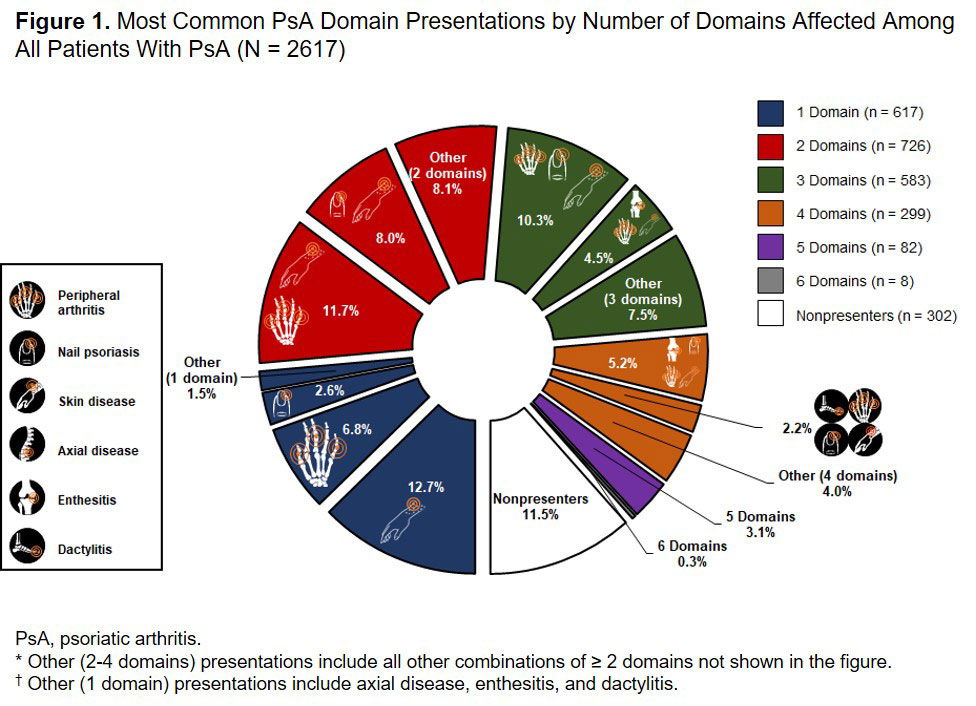
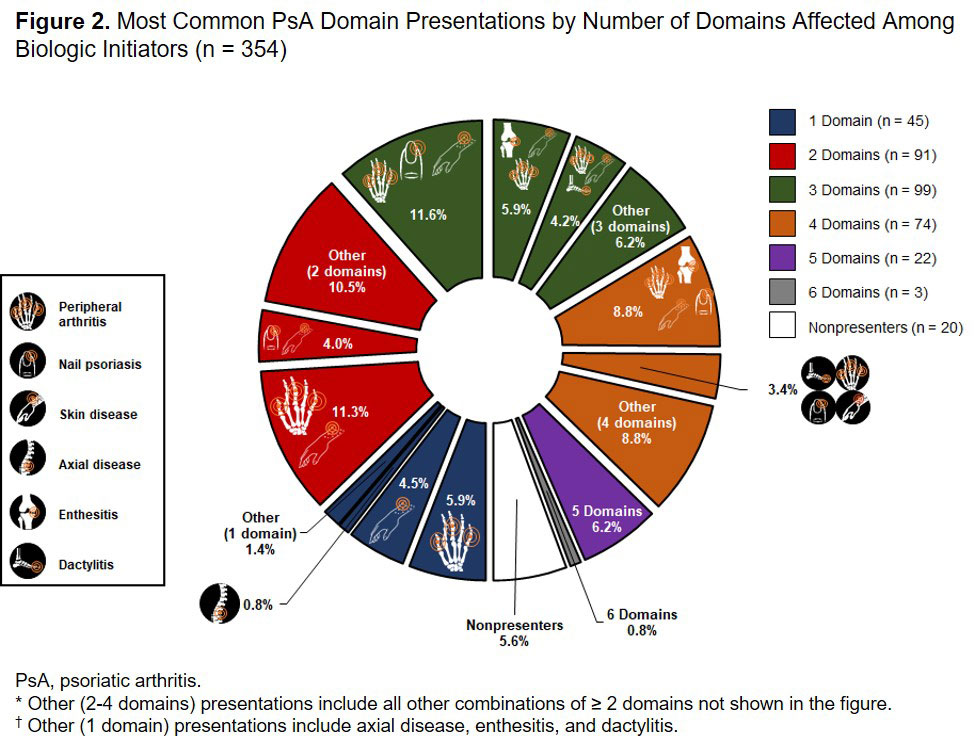
Results: Of 2617 patients with PsA enrolled in the Corrona PsA/SpA Registry, 1698 patients (64.9%) had multidomain disease presentations, 617 (23.6%) had single-domain presentations, and 302 (11.5%) had no presentations. Overall, 1814 (69.3%) patients presented with skin disease, 1523 (58.2%) with PA, 1042 (39.8%) with nail psoriasis, 539 (20.6%) with enthesitis, 319 (12.2%) with axial disease, and 235 (9.0%) with dactylitis at enrollment. Among all patients with PsA, the most common disease presentations were skin disease (12.7%), PA + skin disease (11.7%), and PA + nail psoriasis + skin disease (10.3%) (Figure 1). A total of 354 patients initiated biologics at enrollment. Of these, 289 patients (81.6%) had multidomain disease presentations, 45 (12.7%) had single-domain presentations, and 20 (5.6%) had no presentations; 273 patients (77.1%) presented with PA, 267 (75.4%) with skin disease, 159 (44.9%) with nail psoriasis, 115 (32.5%) with enthesitis, 70 (19.8%) with dactylitis, and 64 (18.1%) with axial disease at enrollment. The most common disease presentations were PA + nail psoriasis + skin disease (11.6%), PA + skin disease (11.3%), enthesitis + PA + nail psoriasis + skin disease (8.8%), and enthesitis + PA + skin disease (5.9%) (Figure 2).
Conclusion: The majority of patients with PsA presented with multiple disease domains. Biologic initiators generally had a higher prevalence of all disease features. These results may increase the physician awareness of the heterogeneity of disease presentations among patients with PsA, which can be important for the development of an effective management plan.
References:
1. Coates LC, et al. Arthritis Rheumatol. 2016;68(5):1060-71.
To cite this abstract in AMA style:
Ogdie A, Hur P, Liu M, Rebello S, McLean R, Dube B, Glynn M, Mease P. Prevalence of Disease Domain Presentations Among Patients with Psoriatic Arthritis: Results from the Corrona Psoriatic Arthritis/Spondyloarthritis (PsA/SpA) Registry [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/prevalence-of-disease-domain-presentations-among-patients-with-psoriatic-arthritis-results-from-the-corrona-psoriatic-arthritis-spondyloarthritis-psa-spa-registry/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-of-disease-domain-presentations-among-patients-with-psoriatic-arthritis-results-from-the-corrona-psoriatic-arthritis-spondyloarthritis-psa-spa-registry/