Session Information
Date: Monday, November 11, 2019
Title: Epidemiology & Public Health Poster II: Spondyloarthritis & Connective Tissue Disease
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: One study estimated that between 161,000 and 322,000 people in the US had definite or probable SLE, based on data from the period of 1965-1973. We aimed to provide current US estimates of SLE prevalence by major health insurance types and describe patient healthcare utilization and characteristics.
Methods: We used 4 large US health insurance claims databases converted to the OMOP Common Data Model: IBM MarketScan® Commercial (CCAE), IBM MarketScan® Medicare Supplemental (MDCR), IBM MarketScan® Multi-state Medicaid (MDCD), and Optum Clinformatics® Data Mart Databases.
In each database, age and sex-specific SLE prevalence was estimated for calendar year 2016.
Numerator for prevalence estimates included individuals with ≥1 SLE diagnosis or ≥1 belimumab prescription in 2016, and met the following before or in 2016:
(1) ≥3 SLE diagnoses (ICD-10 M32.10, or ICD-9 710.0) spanning ≥ 60 days; or
(2) ≥1 belimumab infusion/injection and ≥2 SLE diagnoses; or
(3) ≥1 inpatient SLE diagnosis and ≥1 dispensed prescription for systemic corticosteroids, antimalarials, or immunomodulators commonly used in SLE treatment.
Denominator included individuals enrolled for the entire year of 2016.
Projection of prevalent SLE cases (rounded in thousands) in the US was based on prevalence estimates from each database and US census data by insurance types.
Results: Figure 1 shows the prevalence in females was consistently higher than males across all age groups, ranging from 4- to 23-fold. The highest prevalence in women and men aged 45-64 was observed for those in Medicare who qualify based on disability status. The estimated number of prevalent SLE cases actively treated under commercial (including Medicare supplemental) or Medicaid plans is 450,000 (=285,000+66,000+99,000) to 521,000 (=422,000+99,000) (Table 1). Overall healthcare utilization including hospitalizations for infections appeared highest (despite lowest mean age) in Medicaid (Table 1). Comorbidities were common across multiple organ systems in SLE patients, including renal failure/dialysis. SLE medications most commonly dispensed were systemic corticosteroids and anti-malarial drugs; < 5% of the SLE patients had claims for anti-inflammatory biologic agents.
Conclusion: Estimates of both prevalence proportion and number of civilian SLE cases based on 2016 US data demonstrate a considerable increase over the last three decades, yet likely underestimate the true public health burden which would include persons undiagnosed or not actively seeking healthcare under the insurance plans contributing to our analysis.
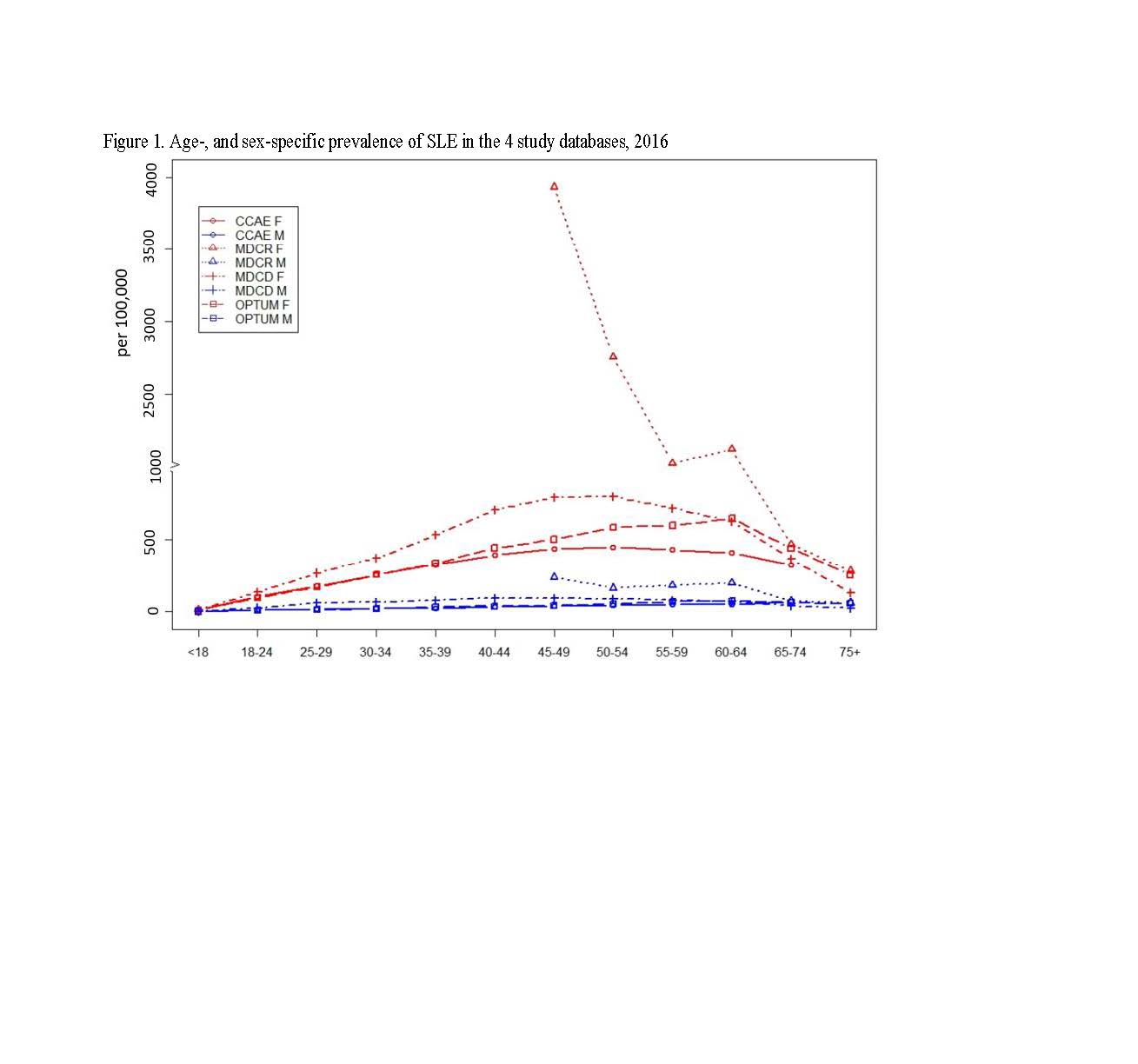
ACR2019_Abs_Figure1_SLE_Epi_and_char_2016US_6.1.19
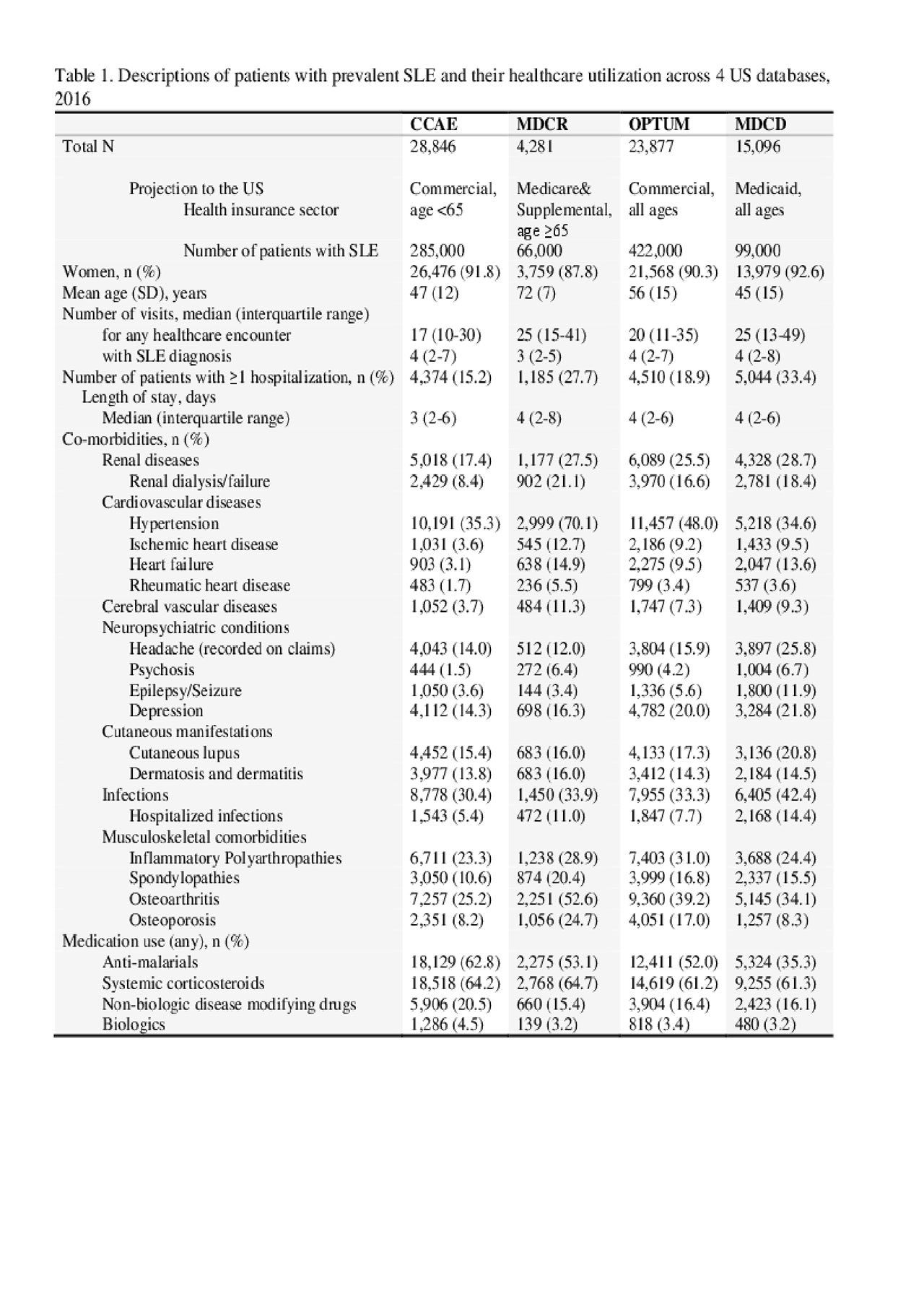
ACR2019_Abs_Table1_SLE_Epi_and_char_2016US_6.3.19
To cite this abstract in AMA style:
Wang Y, Hester L, Lofland J, Rose S, Karyekar C, Kern D, Blacketer M, Davis K, Shields-Tuttle K. Prevalence of Diagnosed Systemic Lupus Erythematosus (SLE), Patient Healthcare Utilization and Characteristics by Major Health Insurance Types in the US [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/prevalence-of-diagnosed-systemic-lupus-erythematosus-sle-patient-healthcare-utilization-and-characteristics-by-major-health-insurance-types-in-the-us/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-of-diagnosed-systemic-lupus-erythematosus-sle-patient-healthcare-utilization-and-characteristics-by-major-health-insurance-types-in-the-us/
