Session Information
Session Type: Abstract Submissions (ACR)
Prevalence of Biologic Utilization over Calendar Time among Medicare Beneficiaries with Rheumatoid Arthritis
Background/Purpose: The establishment of Medicare prescription drug coverage in 2006 and FDA approval of abatacept and rituximab for treatment of rheumatoid arthritis (RA) provided alternative biologics covered under Medicare for RA patients with fee for service coverage. Our objective was to examine the prevalence of biologic use among Medicare beneficiaries with RA from 2006 to 2009.
Methods: Using data from the 100% sample of U.S. Medicare beneficiaries with RA (>= 2 RA diagnoses from rheumatologist visit occurring between 7 and 365 days apart), we identified eligible RA patients with continuous Part A, B, and D coverage for each 3 months interval from July 1st, 2006 to December 31st, 2009; those who had at least one filled prescription or infusion during the 3 months were categorized as users. Among beneficiaries who initiated a biologic after at least 12 months without exposure to any biologic, we performed logistic regression to examine factors associated with the choice of an injection versus an infusion biologic, including demographics, concomitant glucocorticoids and non-biologic DMARDs, calendar year, physician preference, and receiving subsidy for Medicare part B premium from state of residence. Physician preference was measured by the percentage of patients treated with infusion biologics out of all patients treated with biologics in the preceding 6 months by each physician, grouped into quartiles.
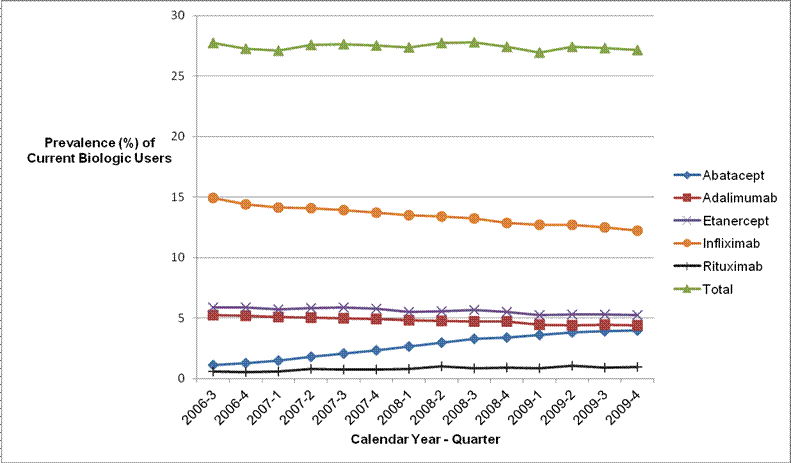
Results: From 2006 to 2009, approximately 27% of all U.S. Medicare beneficiaries with RA treated by rheumatologists received biologics. The prevalence of infliximab use declined from 14.9% in the 3rd quarter of 2006 to 12.2% in the 4th quarter of 2009. In contrast, the prevalence of abatacept increased from 1.1% to 4.0% during this time. We did not observe an increase in the use of self-injectable biologics (Figure 1). After adjusting for covariates, we found that stronger physician preference for infusion biologic was associated with increased odds of infusion biologic use (odds ratio comparing highest quartile to the lowest quartile, 8.9; 95% CI, 8.0-10.0) and receiving subsidized Medicare coverage was associated with increased injection biologic use (odds ratio, 2.8; 95% CI, 2.6-3.0).
Conclusion: The prevalence of injection and infusion biologics remained stable from 2006 to 2009. The choice of infusion versus injection biologics appeared to be strongly driven by patients’ financial considerations and physicians’ preferences.
Disclosure:
J. Zhang,
None;
F. Xie,
None;
E. S. Delzell,
Amgen,
2;
L. Chen,
None;
J. Lewis,
Centocor, Inc.,
2,
Abbott Laboratories, Amgen,
5;
K. Haynes,
Astra Zeneca, BMS,
2;
K. G. Saag,
AHRQ, NIH/NIAMS,
2,
Amgen;Abbott;Ardea:Lilly:Merck:Novartis:Regeneron:Savient:URL,
5,
NOF;ACR,
6;
J. R. Curtis,
Roche/Genetech, UCB< Centocor, Corrona,Amgen, Pfizer, BMS, Crescendo, Abbott, 2, Roche/Genetech,UCB, Centocor, CORRONA, Amgen, Pfizer, BMS, Crescendo, Abbott, 5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-of-biologic-utilization-over-calendar-time-among-medicare-beneficiaries-with-rheumatoid-arthritis/