Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: SLE – Diagnosis, Manifestations, & Outcomes III: Disease Activity
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: There is increasing interest in adopting the principle of treating to target (T2T) in systemic lupus erythematosus (SLE). Remission and low disease activity states have been demonstrated to have protective associations with adverse outcomes such as flare, damage accrual and mortality. However, knowledge gaps remain in relation to the timing and impact of target attainment. In this study, we aimed to identify (i) the proportion of patients achieving the lupus low disease activity state (LLDAS) and the definition of remission in SLE (DORIS-remission) subsequent to a visit with clinically active disease, (ii) time to attainment of LLDAS and DORIS-remission, (iii) determinants of attaining these targets, and (iv) frequency and time to flare and damage accrual following target attainment.
Methods: Patients in the Asia Pacific Lupus Collaboration (APLC) cohort, followed between 2013 and 2020 who had any clinical disease activity according to the clinical domains of SLEDAI-2K and did not fulfill LLDAS or DORIS-remission were followed from their first time fulfilling these criteria. SELENA-SLEDAI flare index and the SLICC/ACR Damage Index were used to assess flare and damage accrual, respectively. We used Multivariable Cox regression models to analyse factors associated with attainment of LLDAS and DORIS-remission. Generalized estimating equation (GEE) models were used to identify factors associated with flare after attainment of target.
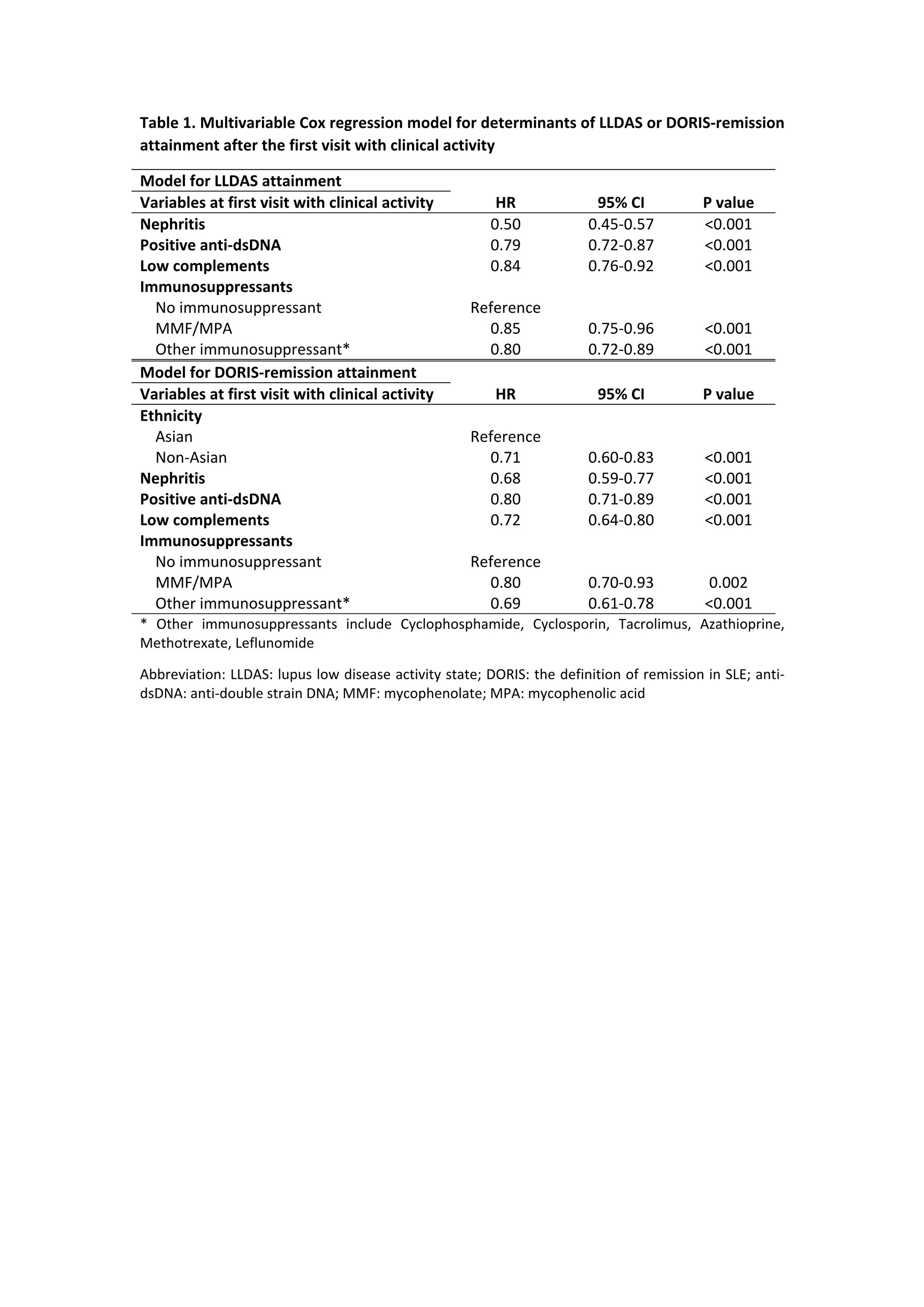
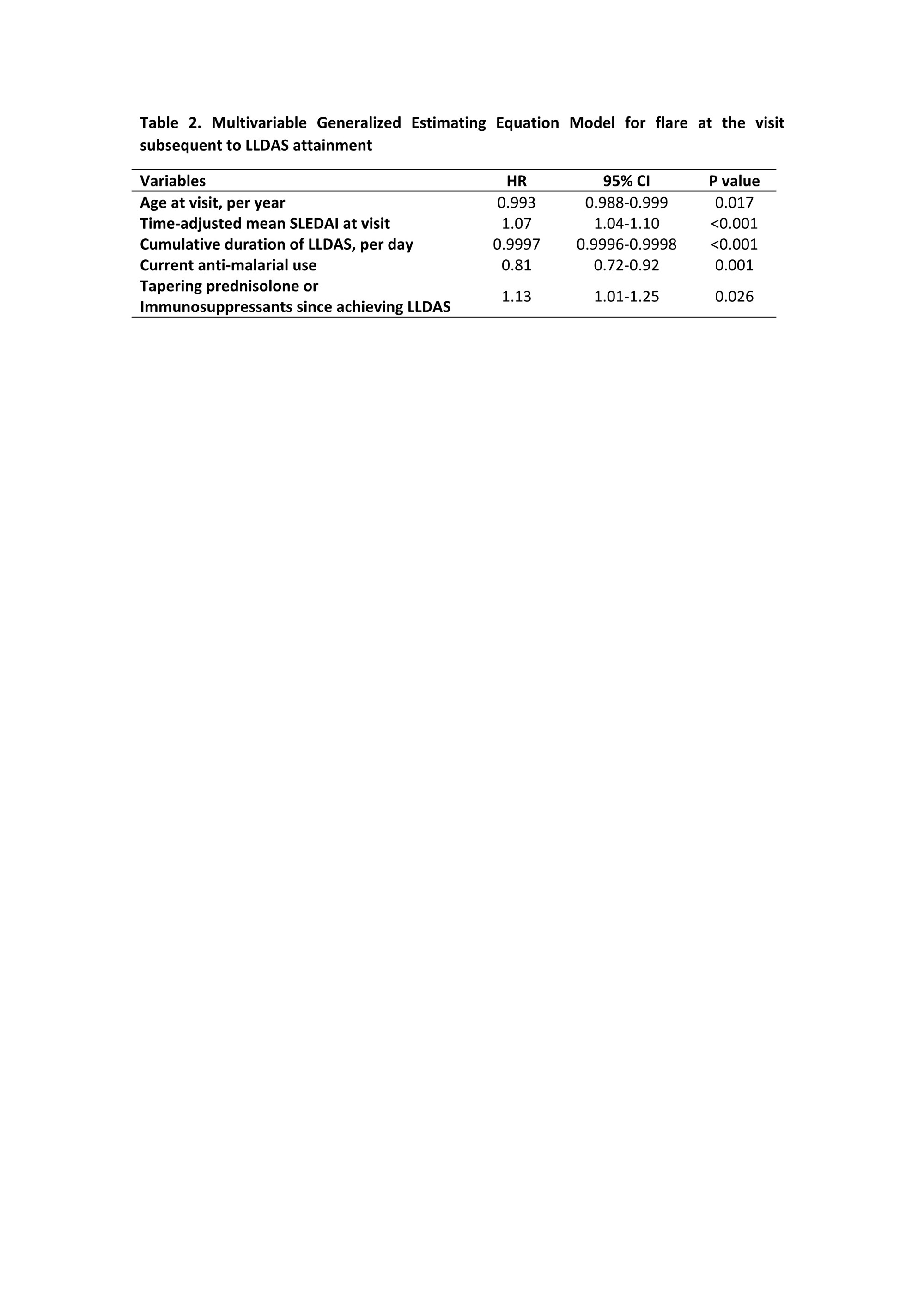
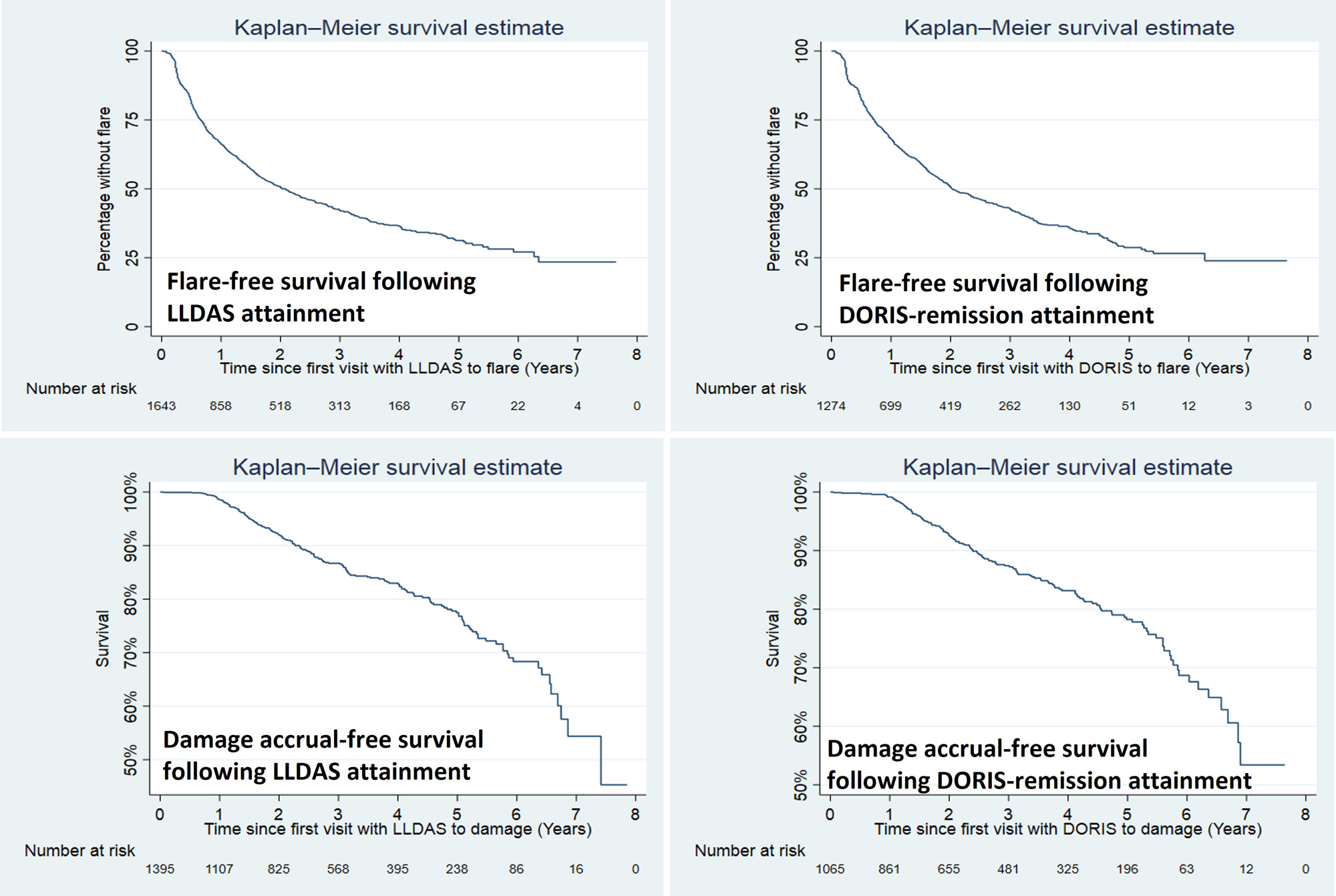
Results: 2852 patients (92.4% female) were followed for a median of 3.3 (1.1-5.7) years. The mean (standard deviations) SLEDAI and PGA at the first visit with clinical activity were 7.0±4.3 and 0.9±0.7, respectively. 1858 (65.2%) of the patients achieved LLDAS, with median (interquartile ranges) time to first attainment of LLDAS being 0.5 (0.3-1.2) years (Figure 1). The proportion and median time to first attainment of DORIS-remission were 49.3% and 0.7 (0.4-1.6) years, respectively. Multivariable Cox model showed that nephritis, positive anti-dsDNA and low complement were associated with a longer time to attainment of LLDAS and DORIS-remission, and in addition non-Asian ethnicity was associated with a longer time taken to attain DORIS-remission (Table 1). After the first attainment of LLDAS, 887/1852 (47.9%) of patients experienced flare(s) with a median time to first flare of 0.7 (0.3-1.4) years. 679/1405 (48.3%) patients had flare(s) after the first attainment of DORIS-remission within a median time of 0.8 (0.3-1.6) years (Figure 1). In multivariable GEE model, factors associated with flare post attainment of LLDAS included tapering of corticosteroids and immunosuppressants (Table 2). 110/960 (11.5%) and 77/750 (10.2%) patients had damage accrual within 24 months of first attainment of LLDAS and DORIS-remission, respectively (Figure 1).
Conclusion: In patients with clinically active disease, LLDAS and DORIS-remission are both achievable goals. However, flares still occur in almost 50% of patients, and 10-11.5% of patients still accrue damage over two years following LLDAS or DORIS-remission attainment, suggesting that further research is needed to inform monitoring and treatment adjustment in SLE patients to maintain states associated with optimal outcomes.
To cite this abstract in AMA style:
Hao Y, Hansen D, Kandane-Rathnayake R, Louthrenoo W, Chen Y, Cho J, Lateef A, Hamijoyo L, Luo S, Wu Y, Navarra S, Zamora L, Li Z, Sockalingam S, Katsumata Y, Harigai M, Zhang Z, Chan M, Kikuchi J, Takeuchi T, Bae S, Goldblatt F, O’Neill S, Ng K, Law A, Basnayake B, Tugnet N, Kumar S, Tee C, Tee M, Tanaka Y, Lau C, Golder V, Hoi A, Morand E, Oon S, Nikpour M. Prevalence, Determinants and Outcomes of Target Attainment in SLE Patients with Clinically Active Disease in a Large Multinational Prospective Lupus Cohort [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/prevalence-determinants-and-outcomes-of-target-attainment-in-sle-patients-with-clinically-active-disease-in-a-large-multinational-prospective-lupus-cohort/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-determinants-and-outcomes-of-target-attainment-in-sle-patients-with-clinically-active-disease-in-a-large-multinational-prospective-lupus-cohort/