Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Tumor necrosis factor alpha inhibitors (TNFi) are used to treat a variety of inflammatory conditions ranging from rheumatoid arthritis to sarcoidosis. Paradoxical side-effects (PSE) of TNFi include the development of conditions normally treated by the therapeutic agent, such as psoriasis and uveitis. Most prevalent among these adverse effects is paradoxical psoriasis, described as having a prevalence of 4.6%1 in patients with inflammatory bowel disease (IBD). Our objective was to validate the prevalence and incidence of PSE in TNFi treatment of rheumatic diseases (RD) patients and to identify risk factors.
Methods: Using our institution’s database on biologic agents in RD, patients that used TNFi were identified. Chart review was performed to identify PSE, which were either documented by the treating clinician or defined as the onset of a condition treatable by the biologic agent (psoriasis, uveitis, hidradenitis suppurativa (HS) or IBD). To identify risk factors, demographic information, diagnosis, and duration of treatment were recorded.
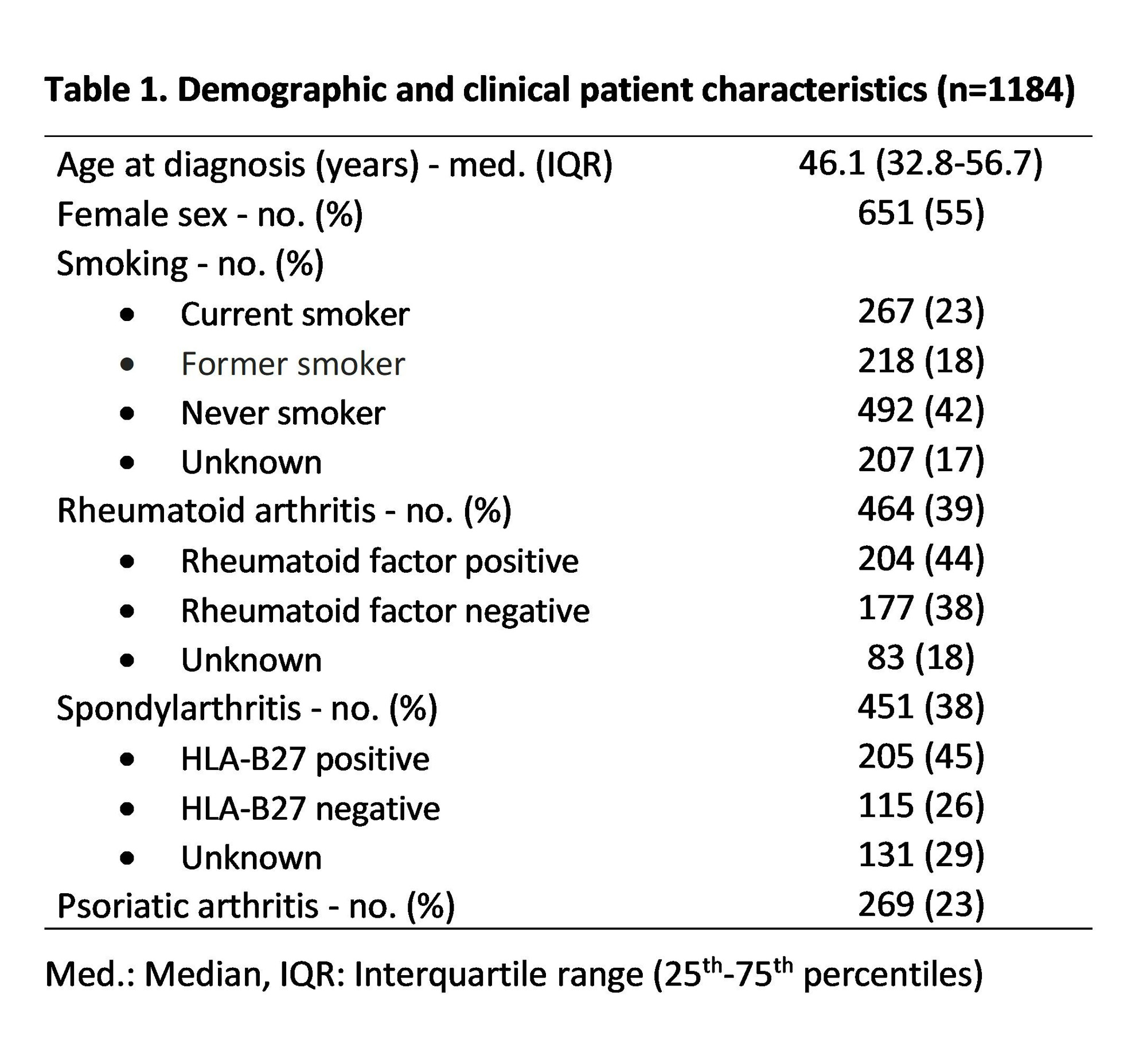
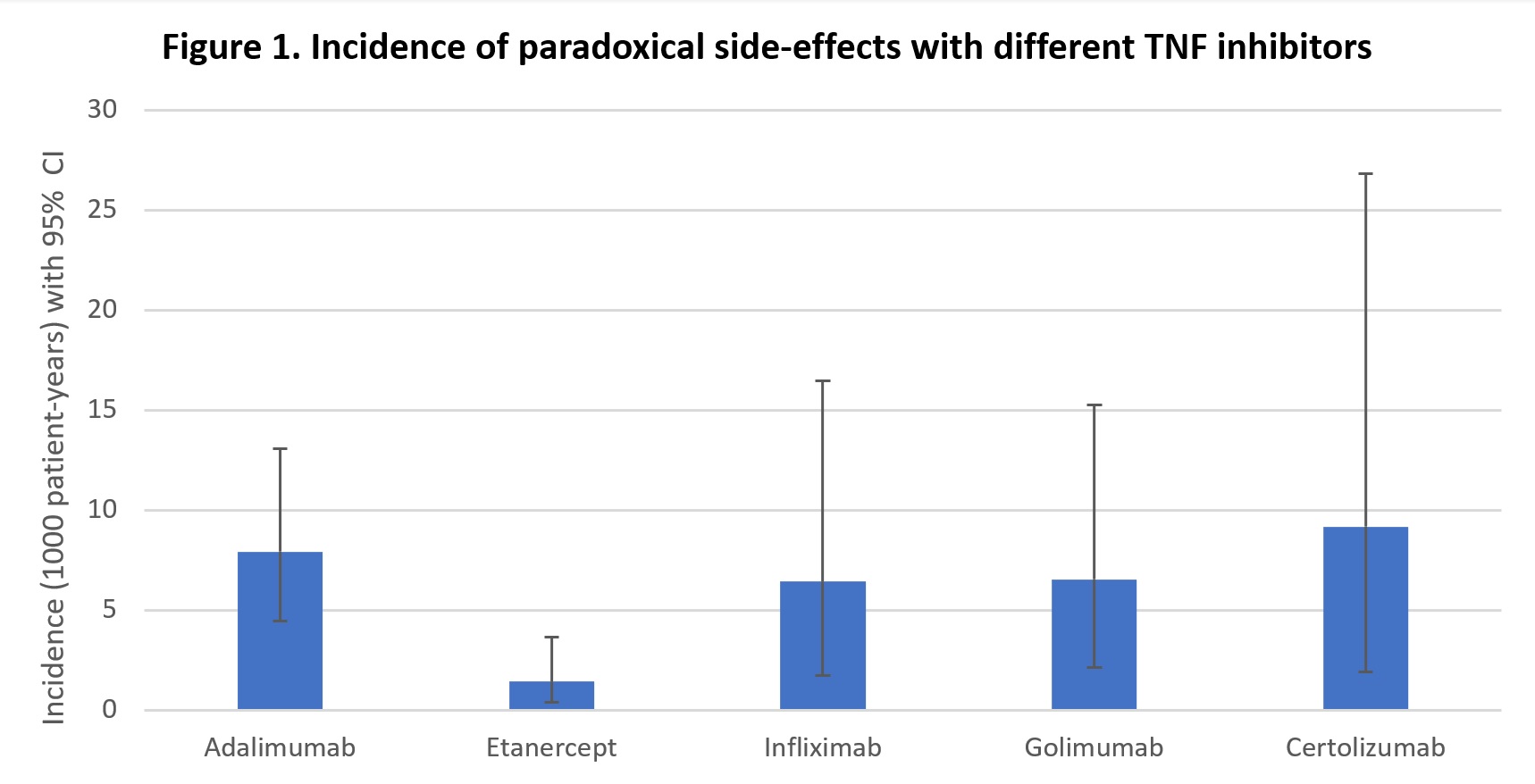
Results: From 2003 to 2023, 1184 patients used at least one TNFi. Median age at diagnosis was 46.1 years old, and females made up 55% of the population. 39% of patients had rheumatoid arthritis while 61% of patients had spondyloarthritides (37% psoriatic arthritis; 63% with other spondylarthropathies). 35 PSE were identified, including 23 cases of psoriasis, 4 cases of Crohn’s disease, 3 psoriasis exacerbations , 2 palmoplantar pustulosis, 2 uveitis and 1 HS. The prevalence of PSE across our population was 3.0% (95% CI 2.1 – 4.1). Patients in the SpA group were three times more likely to experience a PSE when compared to RA with a prevalence of 5.1% vs 1.7% (OR (95% CI) = 3.06 (1.36 – 6.92), p< 0.01). A difference was also observed when comparing prevalence in SpA versus PsA (OR=3.56 (1.22 – 10.41), p=0.02) , while patients younger than 50 years old were more likely to experience PSE (OR=2.34 (1.16 – 2.75), p=0.02).Smoking was a risk factor (OR=2.19 (1.02 – 4.83), p=0.05). Etanercept was less likely to be used in the SpA group compared to the RA group (OR=0.27 (0.20 – 0.36), p< 0.01). Incidence of PSE per 1000 patient-years was 7.9 (95% CI : 4.4 – 13.1) for adalimumab, 2.9 (1.2 – 5.7) for etanercept, 6.4 (1.8 – 16.5) for infliximab, 6.5 (2.1 – 15.3) for golimumab and 9.2 (1.9 – 26.8) for certolizumab. The incidence rate ratio between adalimumab and etanercept was statistically significant (IRR=2.76 (1.10 – 7.52), p=0.02).
Conclusion: Our results identify an overall prevalence of 3.0% for PSE, with a prevalence of 5.1% in SpA, closely matching the prevalence previously reported in IBD. Our results suggest a higher prevalence of PSE in SpA compared to RA. Predisposition to psoriasis in SpA or cutaneous psoriasis appearing after diagnosis of PsA are likely explanations for these results. The use of the decoy receptor, etanercept, compared to monoclonal antibodies was an important protective factor, explained in part by its lesser use in the SpA group. 1. Xie W, Xiao S, Huang H, Zhang Z. Incidence of and Risk Factors for Paradoxical Psoriasis or Psoriasiform Lesions in Inflammatory Bowel Disease Patients Receiving Anti-TNF Therapy: Systematic Review With Meta-Analysis. Front Immunol. 2022;13:847160.
To cite this abstract in AMA style:
Minier A, Boire G, ROUX S, Carrier N, Allard-Chamard H. Prevalence and Incidence of Paradoxical Side-effects of TNF-α Inhibitors: A Cross-sectional Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/prevalence-and-incidence-of-paradoxical-side-effects-of-tnf-%ce%b1-inhibitors-a-cross-sectional-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevalence-and-incidence-of-paradoxical-side-effects-of-tnf-%ce%b1-inhibitors-a-cross-sectional-study/