Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: B-cell targeted therapeutics have proven efficacious in the treatment of autoimmune disorders. A desired improvement in efficacy and safety necessitate the development of alternate, fast acting and non-depleting B cell modulators. PRV-3279 (previously MGD010) a bispecific molecule, exploits the inhibitory function of the SLE-associated checkpoint molecule, CD32B, (FcγRIIb) via its simultaneous co-ligation with the BCR component, CD79B. Initial single-dose Phase 1 trials in healthy subjects demonstrated PRV-3279 was well tolerated and modulated B cells without depletion. PREVAIL (PRV-3279 EVAluation In Lupus) 1, a multiple ascending dose study in healthy subjects, was performed to further characterize the safety, PK and PD of PRV-3279 upon repeat administration. In vitro studies on B cells from SLE subjects were also performed to support transition into the planned Phase 2a PREVAIL 2 study in SLE.
Methods: This was a double-blind, placebo-controlled MAD study in healthy volunteers. Subjects (n=8 per group) were randomly assigned to two cohorts to receive an IV administration of 3 bi-weekly infusions of 3 or 10 mg/kg PRV-3279, or placebo (6:2). Safety, PK, binding of PRV-3279 to total and B cell sub-sets, peripheral lymphocyte counts, and determination of serum immunoglobulin levels were assessed for 12 weeks. Additionally, CD32B and CD79B levels on purified B cells from SLE subjects and the effect of PRV-3279 on SLE B cell proliferation was determined, compared with healthy controls.
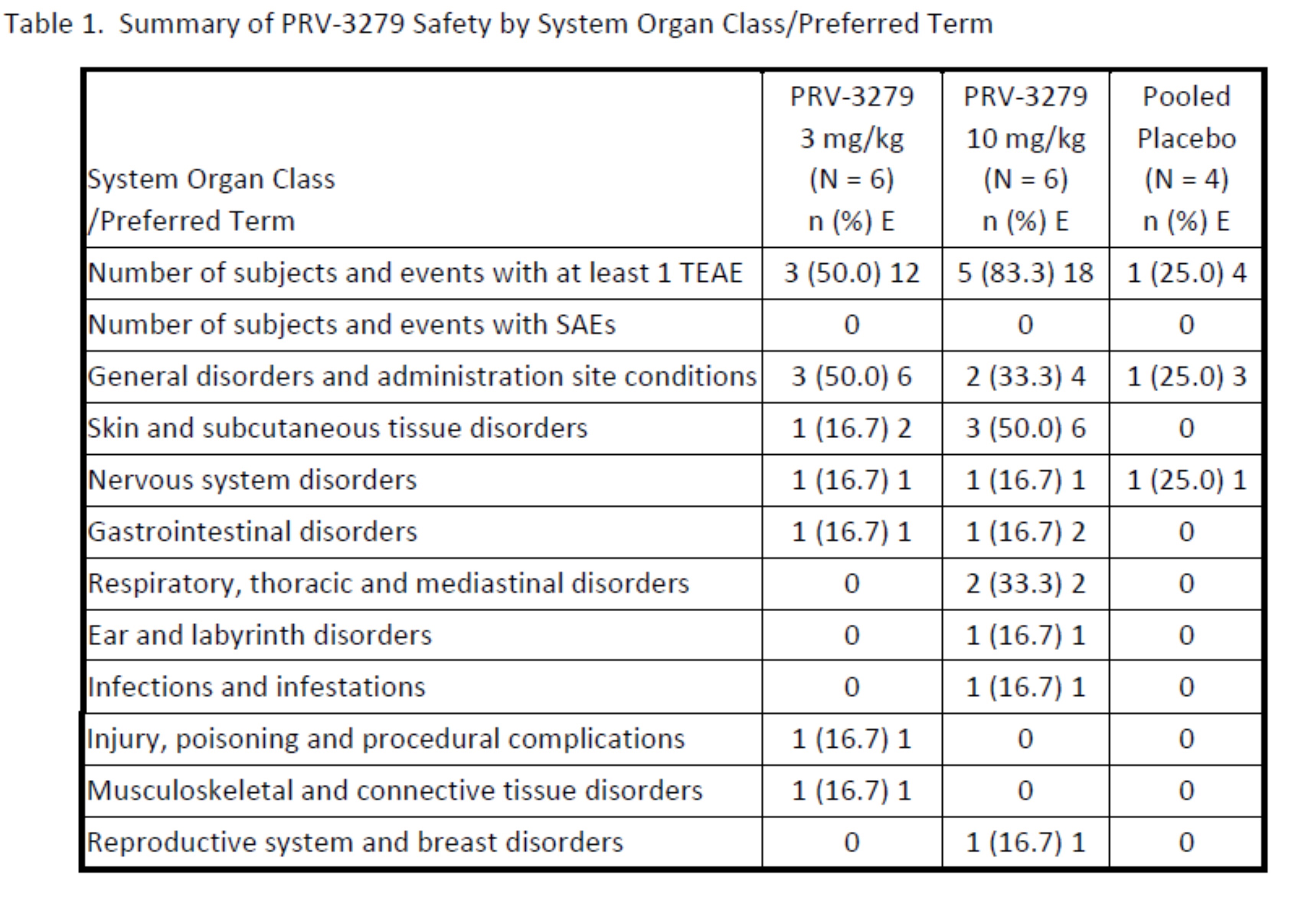
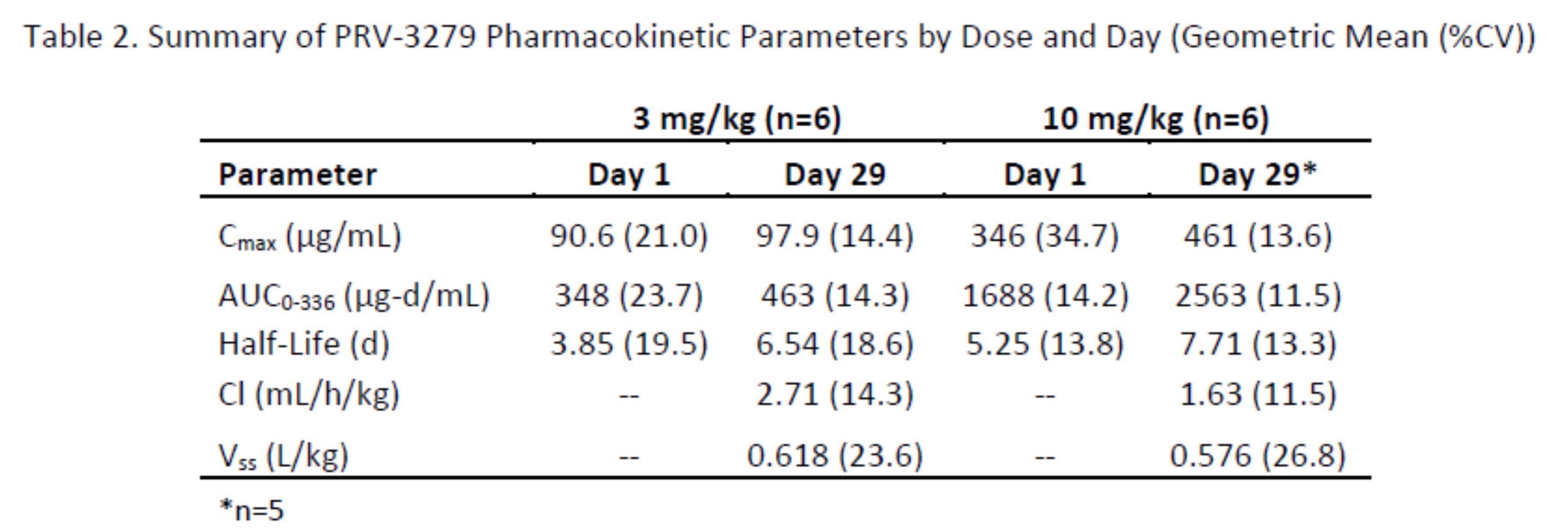
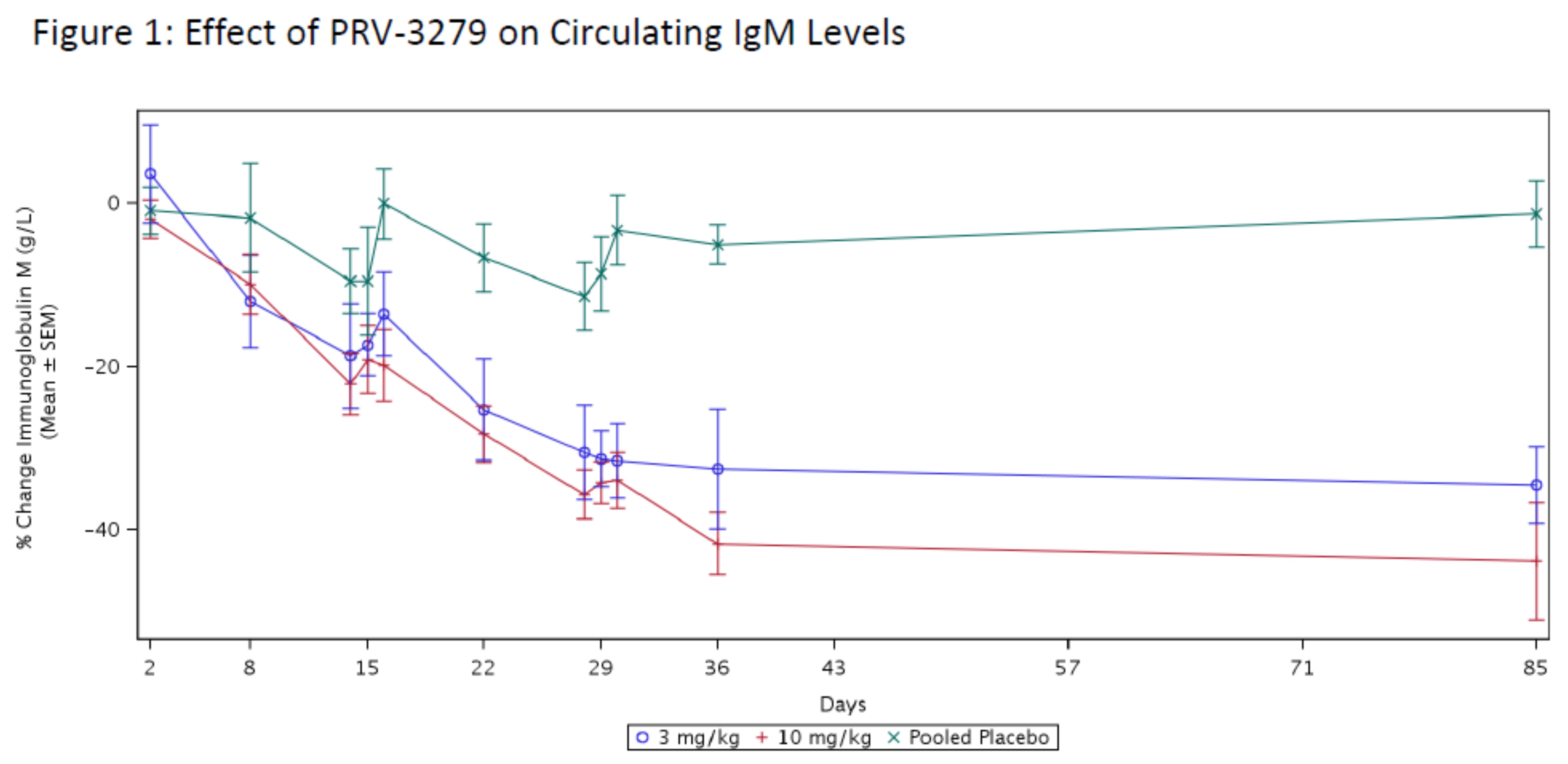
Results: PRV-3279 was well-tolerated, with no serious adverse events (Table 1). Pharmacokinetic (Table 2) and pharmacodynamic parameters were dose-dependent with extensive binding to both naïve and memory B cells, and a mean sustained binding of >90% available B cells observed at 10 mg/kg. An extended pharmacodynamic effect was demonstrated by a sustained and maximum mean reduction of 44% in circulating IgM levels at 10 mg/kg, at the final sampling at Week 12 (Figure 1). PRV-3279 did not deplete B cells, although there was a mean transient decrease of up to 47% after each infusion, returning to baseline at the next measurement (+1 week). In vitro, expression of CD32B and CD79B on SLE patient B cells was similar to healthy controls, and PRV-3279 inhibition of SLE B cell proliferation (mean 63% reduction) was not significantly different to those from healthy controls.
Conclusion: PRV-3279 was well tolerated after repeat administration with no B cell depletion and dose-dependent PK and PD. Consistent with its mechanism of action, sustained IgM reduction was observed. In vitro studies demonstrated equivalent effects of PRV-3279 on B cells from SLE subjects. These data support further development of PRV-3279 in SLE and other autoimmune disorders.
To cite this abstract in AMA style:
Dunford P, Comer G, Raymond R, Jung D, Moore P, Leon F, Merrill J. PREVAIL 1: A Multiple Ascending Dose Study in Normal Healthy Volunteers of PRV-3279, a Novel Bispecific DART Molecule Targeting CD32B and CD79B on B Cells, with Potential for Treatment of SLE [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/prevail-1-a-multiple-ascending-dose-study-in-normal-healthy-volunteers-of-prv-3279-a-novel-bispecific-dart-molecule-targeting-cd32b-and-cd79b-on-b-cells-with-potential-for-treatment-of-sle/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prevail-1-a-multiple-ascending-dose-study-in-normal-healthy-volunteers-of-prv-3279-a-novel-bispecific-dart-molecule-targeting-cd32b-and-cd79b-on-b-cells-with-potential-for-treatment-of-sle/