Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept, a selective T-cell co-stimulation modulator, significantly increased ACR20 response and had an overall beneficial effect on musculoskeletal symptoms in patients with active psoriatic arthritis (PsA) in the Phase III Active pSoriaTic athRitis rAndomizEd triAl (ASTRAEA, NCT01860976).1 Factors that may predict responses to abatacept were explored in this post hoc analysis. The aim of this study was to evaluate the relationship between baseline characteristics and abatacept response in a post hoc analysis of ASTRAEA.
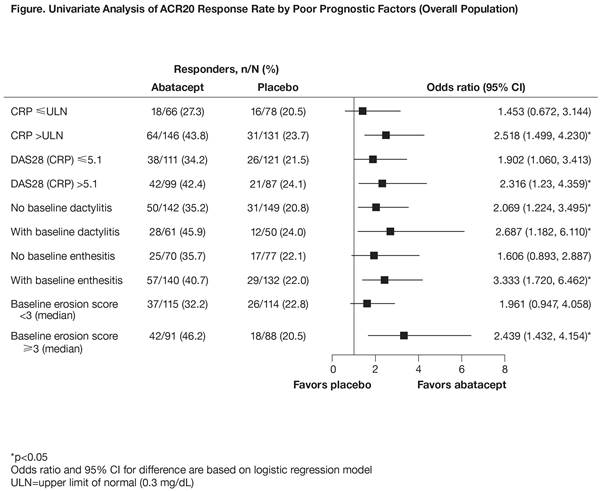
Results: Of 424 patients enrolled, 213 received abatacept and 211 placebo. In abatacept-treated patients, the multivariate model showed a difference in ACR20 response (OR >1.2) for baseline CRP (>upper limit of normal [ULN] vs ≤ULN; OR 1.346 [95% CI 0.668, 2.712]), DAS28 (CRP) (>5.1 vs ≤5.1; 1.489 [0.782, 2.836]), dactylitis (>0 vs 0; 1.372 [0.708, 2.659]), and median baseline erosions (≥3 vs <3; 1.924 [1.032, 3.587]). In placebo-treated patients, the OR was >1.2 for dactylitis only (1.406 [0.619, 3.193]). These factors, which have been identified previously as indicating poor prognosis in PsA, were balanced between treatment arms at baseline. In the univariate model by poor prognostic factors, the differences in ACR20 response rates with abatacept treatment vs placebo in distinct subgroups were numerically greater in patients who were positive for these prognostic factors at baseline than in those who were not (Figure).
Conclusion: These findings identified subgroups of patients with PsA with certain baseline characteristics in whom abatacept is most likely to be effective. The predictive factors identified are aligned with poor prognostic factors in the EULAR and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) guidelines,2,3 and may indicate patients with the highest unmet medical need.
1. Mease P, et al. Arthritis Rheumatol 2016;68(Suppl 10):Abstract 1041.
2. Gossec L, et al. Ann Rheum Dis 2016;75:499–510.
Original abstract © EULAR/BMJ. First presented at EULAR 2017 and published in Ann Rheum Dis 2017;76 (Suppl 2):SAT0468. Any reprints, promotional options, education material etc have to be done through the original source (ARD/BMJ).
To cite this abstract in AMA style:
Mease PJ, McInnes IB, Strand V, FitzGerald O, Ahmad H, Johnsen A, Ye J, Banerjee S. Presence of Poor Prognostic Factors May Predict Response to Abatacept in Patients with Active Psoriatic Arthritis: Results from a Post Hoc Analysis from a Phase III Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/presence-of-poor-prognostic-factors-may-predict-response-to-abatacept-in-patients-with-active-psoriatic-arthritis-results-from-a-post-hoc-analysis-from-a-phase-iii-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/presence-of-poor-prognostic-factors-may-predict-response-to-abatacept-in-patients-with-active-psoriatic-arthritis-results-from-a-post-hoc-analysis-from-a-phase-iii-study/
