Session Information
Date: Monday, November 6, 2017
Title: Health Services Research Poster II: Osteoarthritis and Rheumatoid Arthritis
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
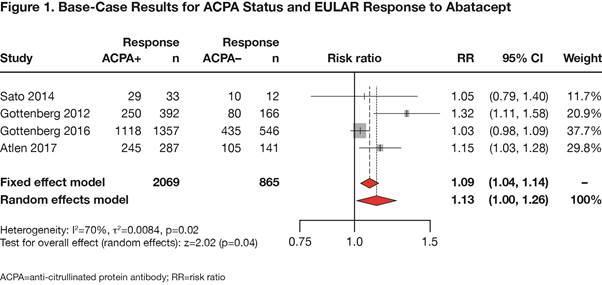
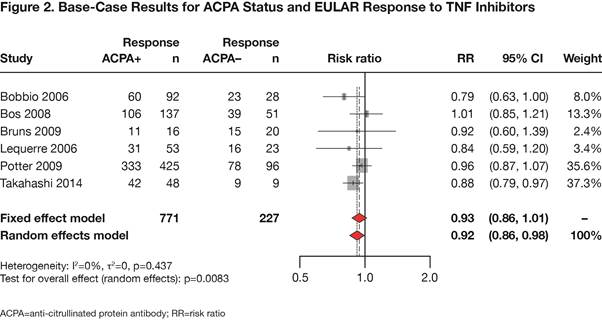
Conclusion: This meta-analysis confirms that ACPA-positive RA patients are more likely to achieve response to abatacept treatment compared to ACPA-negative patients. Additionally, the analysis demonstrates that there is no association between ACPA status and response to TNFi therapy, consistent with the findings of previously published studies.1,3
To cite this abstract in AMA style:
Alemao E, Postema R, Elbez Y, Mamane C, Finckh A. Presence of Anti-Cyclic Citrullinated Peptide Antibodies Is Associated with Better Treatment Response to Abatacept but Not to TNF Inhibitors in Patients with RA: A Meta-Analysis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/presence-of-anti-cyclic-citrullinated-peptide-antibodies-is-associated-with-better-treatment-response-to-abatacept-but-not-to-tnf-inhibitors-in-patients-with-ra-a-meta-analysis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/presence-of-anti-cyclic-citrullinated-peptide-antibodies-is-associated-with-better-treatment-response-to-abatacept-but-not-to-tnf-inhibitors-in-patients-with-ra-a-meta-analysis/