Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Colchicine, a commonly used drug, had never been subjected to rigorous safety and efficacy studies prior to 2009, when under Hatch-Waxman ACT (1984) and the Unapproved Drug Initiative, the Food and Drug Administration approved a proprietary version of colchicine (Colcrys®) for treatment of acute gout flare. The approved dose was much lower than that used traditionally. One assumption for the FDA approval of colchicine was that proprietary versions of the previously unapproved products would enhance patient safety by encouraging more rational prescribing practices. The goal of this study was to test this assumption using clinical data.
Methods: The data used for the present study was retrieved from the STRIDE (Stanford Translational Research Integrated Database Environment) database that includes electronic medical record data from 15 million clinical encounters with 1.6 million unique patients. After validating diagnosis of gout by chart review we identified 970 records of gout utilizing colchicine from 2006 to 2012. High-dose colchicine (HDC) and very-high-dose colchicine (VHDC) prescriptions were defined as more than 1.8 mg/day and 3.0 mg/day respectively. Factors associated with HDC and VHDC were analyzed using logistic regression models. Although Colcrys ® was approved in June 2009 we assumed that the earliest time for discernible impact to be January 1, 2010.
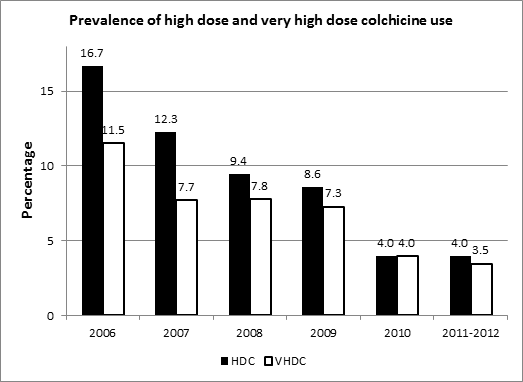
Results: Before and after 2010, the mean age and mean serum creatinine were similar, while the proportion of women and mean serum uric acid were statistically different (Table). The proportion of patients using HDC/VHDC decreased from 11.7%/8.6% before 2010 to 4.0%/3.7% after 2010, representing an unadjusted odds ratio of 0.3 (0.2, 0.6)/0.4 (0.2, 0.8) and age-sex-adjusted odds ratio of 0.3 (0.2, 0.6)/0.4 (0.2, 0.8). The utilization of high-dose colchicine has been declining steadily even before the introduction of Colcrys®, while the utilization of very-high-dose colchicine declined after 2007 and was steady through 2007 to 2009 and then declined after 2010(Figure). In multivariable logistic regressions that adjusted for age, sex and renal function, the odds ratio for HDC/VHDC 2010-2012 compared to the preceding years (2006-2009) was 0.4 (0.2, 0.6)/0.5 (0.2, 0.9).
Conclusion: Colchicine dosing has been declining over time. Statistically this is unrelated to the approval of Colcrys® under the FDA Unapproved Drug Initiative.
|
|
2006-2009 |
2010-2012 |
|
Mean Age in years (SD) |
65.7 (17.8) |
67.3 (14.8) |
|
Proportion of women* |
17.9% |
23.4% |
|
Mean serum creatinine in mg/dL (SD) |
1.3 (1.0) |
1.4 (1.4) |
|
Mean serum uric acid in mg/dL (SD)* |
7.3 (2.8) |
6.4 (2.5) |
SD: standard deviation; * Statistically different (p<0.05)
Figure
Disclosure:
E. Krishnan,
takeda,mboles,Ardea,
2,
Takeda,Metabolex,
5;
J. A. Singh,
None;
L. Chen,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prescription-patterns-of-colchicine-before-and-after-food-and-drug-administration-approval/