Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with polymyalgia rheumatica (PMR) and giant cell arthritis (GCA) constitute approximately 20% of diseases chronically treated with glucocorticoids (GC) with a daily dose of 7.5 mg or more,1 and GC-related adverse events (AE) are reported in up to 65% of PMR patients.2 Additionally, 50% of secondary care patients are not able to discontinue their GC treatment, emphasizing the need for GC-sparing agents.3 The recent guidelines on management of PMR recommend early introduction of methotrexate (MTX), especially in patients at risk for worse prognosis such as female gender, high erythrocyte sedimentation rate (ESR) and peripheral arthritis at diagnosis.4 However, evidence regarding MTX use in daily clinical practice is limited.4 Therefore our aim is to assess MTX treatment in GC dependent PMR patients in a large Dutch rheumatology clinic.
Methods: This is a retrospective cohort study of newly diagnosed PMR patients (clinical diagnosis) who visited our rheumatology clinic between April 2012 and September 2017. Patients with concomitant active inflammatory rheumatic disease were excluded. Data on patient, disease and treatment characteristics were extracted from the electronic health records. Descriptive statistics were used as appropriate.
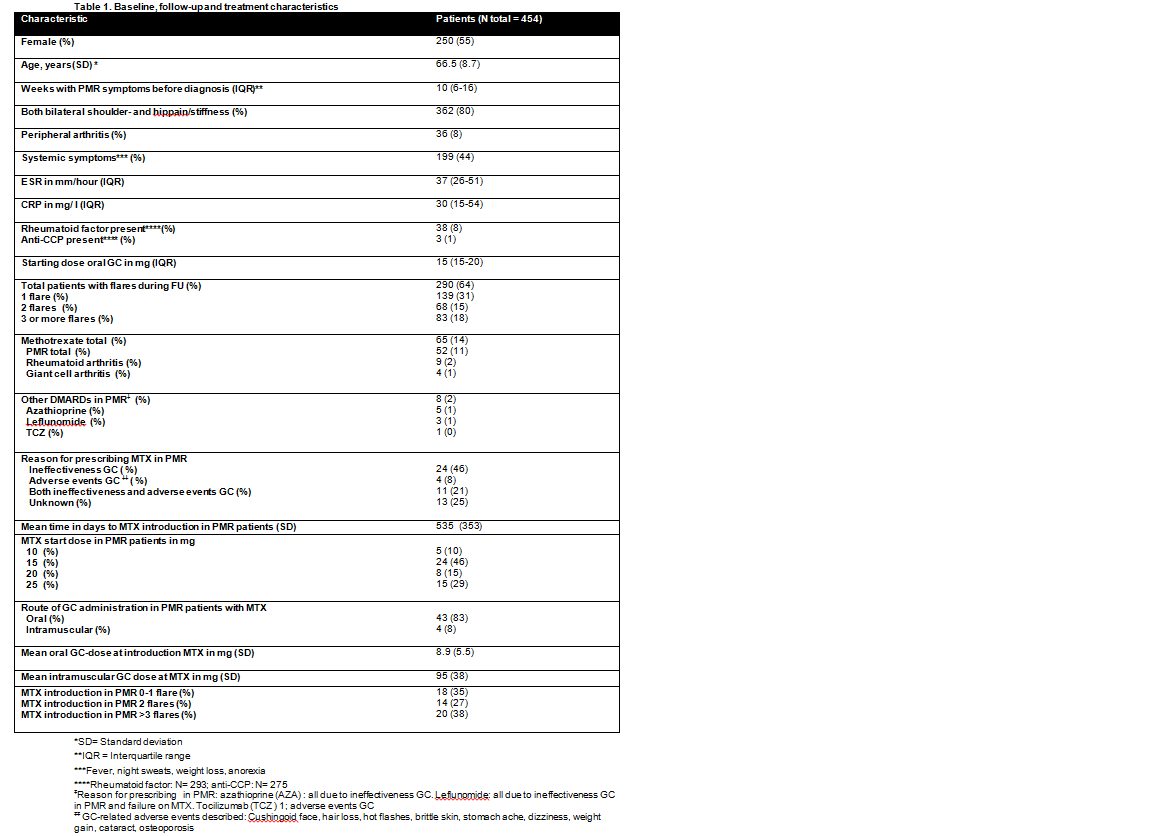
Results: Baseline, follow-up and treatment characteristics of the 454 included patients are described in table 1. MTX was prescribed in 52/454 (11%) PMR patients while 33% of patients had two or more flares during follow-up. Other disease modifying anti-rheumatic drugs (DMARDs) were prescribed in 2% of patients. Reasons for prescribing MTX were GC ineffectiveness in 46%, GC adverse events in 8%, both GC ineffectiveness and adverse events in 21% and unknown in 25% of patients. Mean time to MTX introduction was 535 days (SD 353) and the starting MTX dose was 10mg in 10%, 15 mg in 46%, 20mg in 15% and 25mg in 29% of PMR patients respectively. Of the PMR patients treated with MTX, 83% was on oral GC (mean dose was 8.9mg, SD 5.5mg) and 8% on intramuscular GC (mean dose 95mg per approximately one month, SD38mg). At the start of MTX treatment, 38% of patients had experienced three or more flares, 27% two flares and 35% one flare.
Conclusion: Early introduction of concomitant MTX early in GC dependent PMR is limited in our rheumatology clinic. Several interventions can be conceived to enhance follow-up to (inter)national guidelines on management of PMR.
References
- L. Couvaras et al. Prevalence of long-term steroid therapy: French data. AB1141 (2017)
- González-Gay MA et al.. Polymyalgia rheumatica. Lancet. 2017 Oct Oct 7;390(10103):1700-1712
- Albrecht, K. et al. Rheumatol Int (2018) 38: 569. https://doi.org/10.1007/s00296-017-3874-3
- Dejaco C et al. 2015 Recommendations for the management of polymyalgia rheumatica: a European League Against Rheumatism/American College of Rheumatology collaborative initiative.Annals of the Rheumatic Diseases 2015;74:1799-1807.
To cite this abstract in AMA style:
Marsman D, van der Maas A, den Broeder N, Bolhuis T, Boers N, den Broeder A, van den Hoogen F. Prescribing Methotrexate in Polymyalgia Rheumatica: A Missed Opportunity? [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/prescribing-methotrexate-in-polymyalgia-rheumatica-a-missed-opportunity/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/prescribing-methotrexate-in-polymyalgia-rheumatica-a-missed-opportunity/