Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown etiology with mortality still approaching 10% in 5 years. The major cause of deaths in SLE include infections due to the toxicity of immunosuppressant medications. Our study design has been formulated on the premise that safe and effective treatment should target key checkpoints of pathogenesis. The depletion of cysteine and reduced glutathione (GSH) underlie mitochondrial oxidative stress, activation of the mechanistic target of rapamycin (mTOR), inflammatory lineage specification and antinuclear autoantibody production in SLE. An earlier completed double-blind, placebo-controlled study provided preliminary evidence that replacement of cysteine and GSH with amino acid precursor, N-acetylcysteine (NAC), can safely reduce mTOR activation, anti-DNA production, and disease activity in SLE (PMID: 22549432; PMID: 23400548).
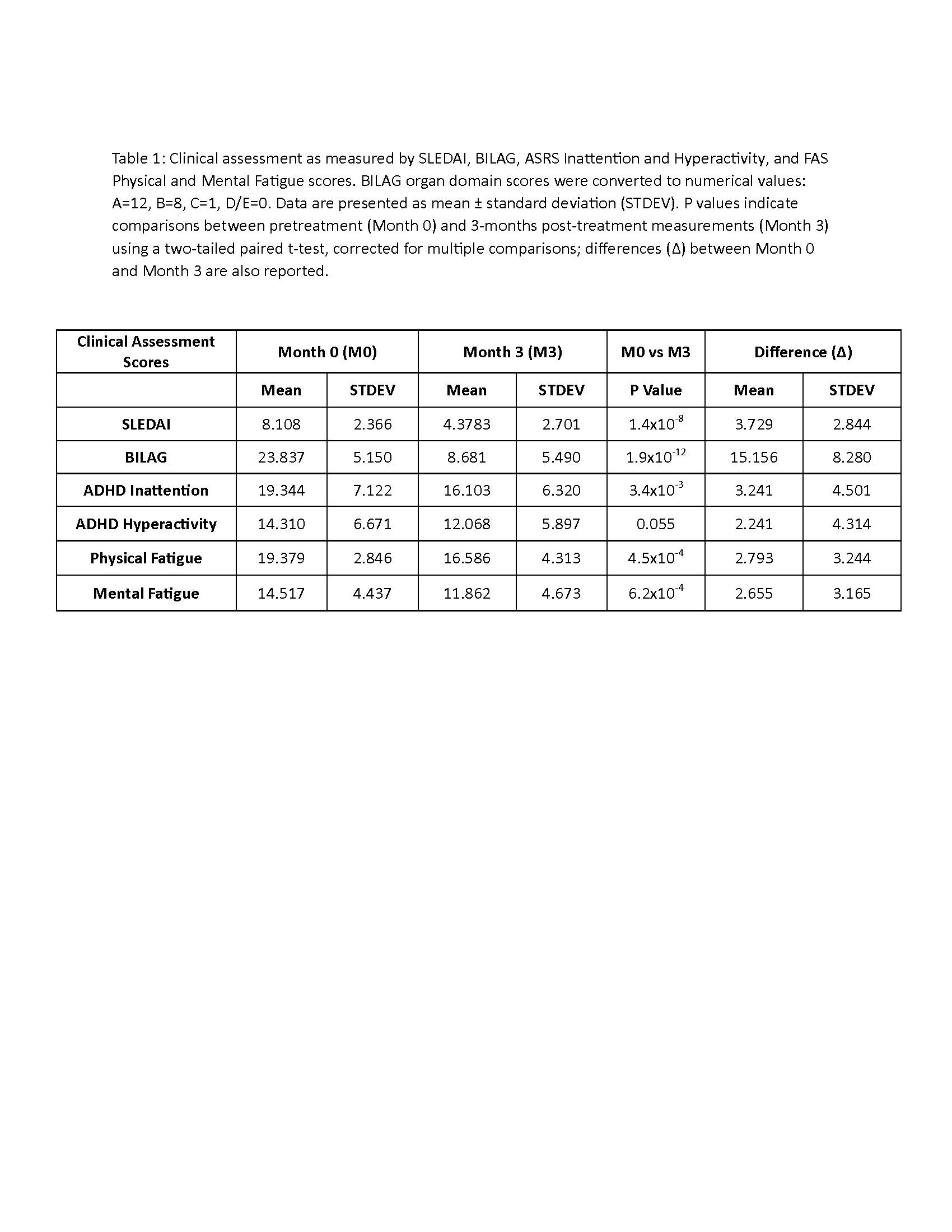
Methods: The study is a multicenter randomized, double-blind, placebo-controlled clinical trial, using an innovative enriched enrollment randomized withdrawal (EERW) design that allows participants to use an individualized dosage of study medication, which mimics clinical practice in terms of how NAC would be administered. Eligibility criteria required clinically active disease with ≥6 SLE Disease Activity Index (SLEDAI) and British Isles Lupus Assessment Group (BILAG) organ domain scores ≥1 BILAG A or ≥2 BILAG B (clinicaltrials.gov NCT00775476). All participants received NAC for 3 months, titrated up to a maximally tolerated dosage between 2.4 g/day and 4.8 g/day. Subjects tolerating ≥2.4 g/day NAC have been randomized to receive either NAC or placebo at the subject’s tolerated dosage for the remaining 9 months of treatment. Our preliminary analysis evaluated the BILAG and SLEDAI scores in 37 patients, and validated Fatigue Assessment (FAS) and ADHD Self-Report Scales (ASRS) (PMID: 23400548) in 29 SLE patients, before and after a 3-month open-label dose titration phase. Statistical analysis included two-tailed paired t-tests and correlations corrected for multiple comparisons using the Bonferroni correction in GraphPad software.
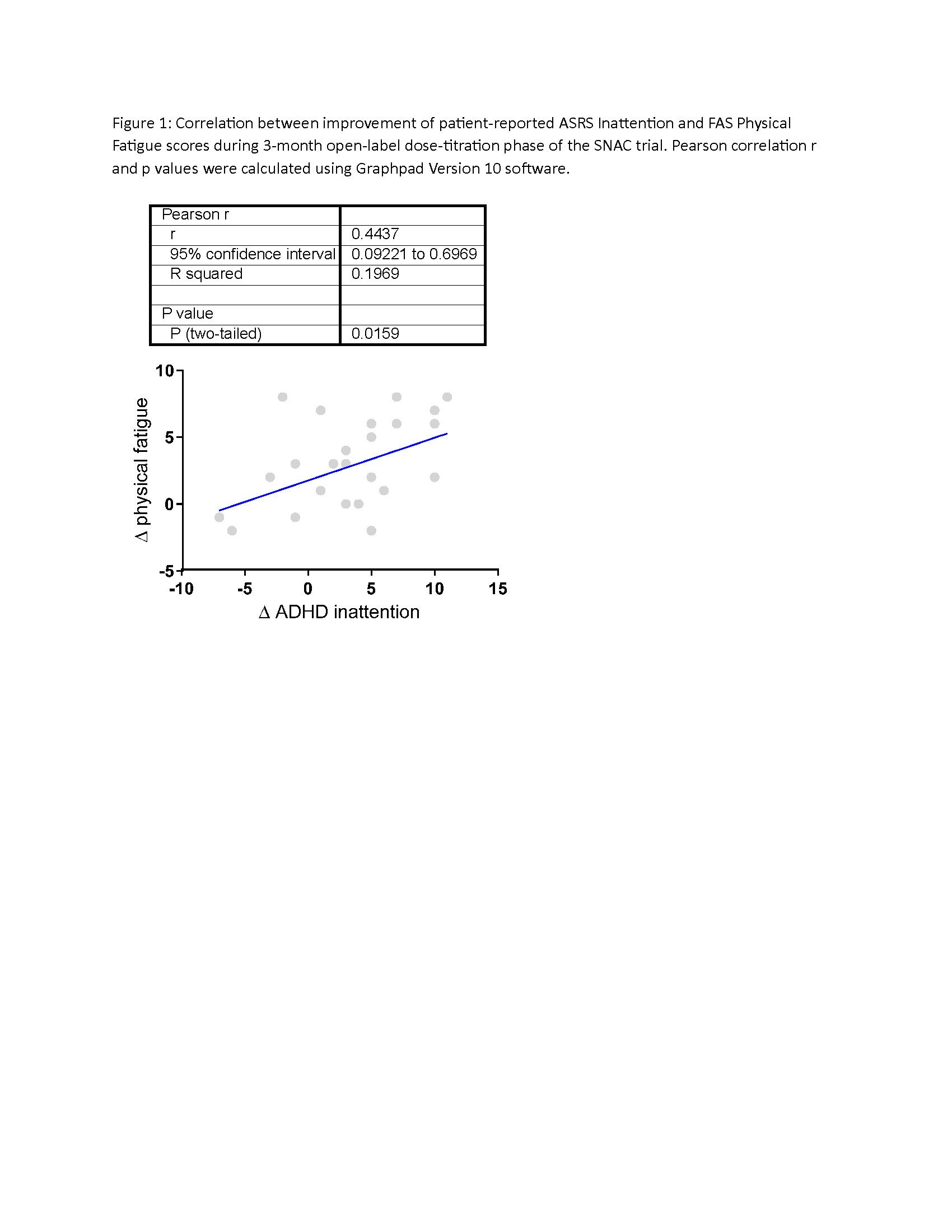
Results: We observed significant reduction of SLE disease activity as measured by the reduction of SLEDAI and BILAG muco-cutaneous and musculoskeletal organ domain scores. ASRS cognition/inattention and fatigue scores also improved (Table 1). Improvement of inattention correlated with that of physical fatigue (Figure 1). Adverse events, all rapidly reversible, included headaches, nausea, upset stomach, constipation and diarrhea, which resulted in dosage reduction in 6 patients and dropout in 4 patients out of a total of 10 dropouts by month 3.
Conclusion: This preliminary analysis supports the notion that NAC may serve as a safe and effective treatment for patients with SLE, but these observations could be affected by the absence of blinding in the open-label phase and regression-to-the-mean. The double-blind phase of the ongoing multicenter trial will determine whether NAC is efficacious in treating SLE.
To cite this abstract in AMA style:
Ruchi F, Coman I, Blaker B, Blaker L, Park J, Cabezas J, Wang X, Godavarthy A, Marte Furment M, Nasr S, Lokineni S, Donath C, Kahlown S, Sereda D, Weinstein A, Weisman M, Aranow C, Ishimori M, Kirou K, Yu J, Ben Gabr J, Rayancha S, Olsen N, Faraone S, Rao D, Ramsey-Goldman r, Wallace D, McDermott M, Perl A. Preliminary Analysis of Open-Label Dose-Titration Phase of SLE Treatment with N-Acetylcysteine (SNAC) Shows Evidence for Potential Improvement of SLEDAI, BILAG, ADHD and Fatigue Scores in Patients with Active SLE [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/preliminary-analysis-of-open-label-dose-titration-phase-of-sle-treatment-with-n-acetylcysteine-snac-shows-evidence-for-potential-improvement-of-sledai-bilag-adhd-and-fatigue-scores-in-patients-wit/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-analysis-of-open-label-dose-titration-phase-of-sle-treatment-with-n-acetylcysteine-snac-shows-evidence-for-potential-improvement-of-sledai-bilag-adhd-and-fatigue-scores-in-patients-wit/