Session Information
Date: Sunday, November 10, 2019
Title: Pediatric Rheumatology – ePoster I: Basic Science, Biomarkers, & Sclerodermic Fever
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Neonatal-onset multisystem inflammatory disease (NOMID), caused by gain-of-function mutation in the NLRP3 inflammasome, presents with systemic inflammation, rash, eye inflammation, aseptic meningitis and sensorineural hearing loss. We assessed hearing loss progression in NOMID patients on anakinra treatment over median of 10 years.
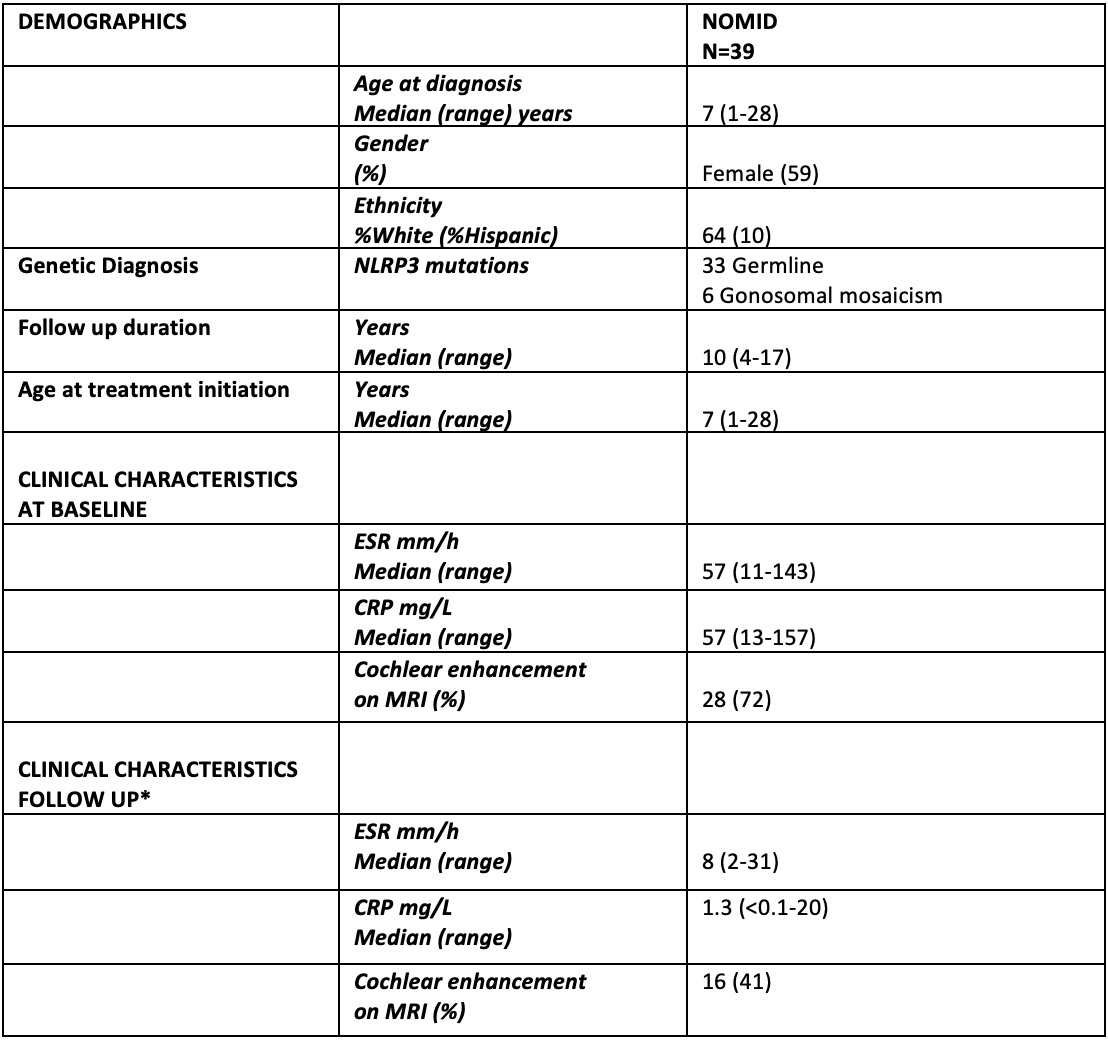
Methods: 39 patients (23 female, median age at baseline 7y) were followed (median 10y) after starting therapy with IL-1 blocker Anakinra (table 1). All patients were on continuous therapy with dose adjustment for weight gain and disease flare (1). First audiograms obtained at the time of treatment initiation (or when able to cooperate) were compared to an audiogram obtained after a median of 10 (4 to 17) years on treatment. Pure-tone averages at 500, 1000, 2000, 4000 Hz (4F-PTA) and high frequency air conduction averages for 6000, 8000 Hz (HF-PTA) were calculated. For the current analysis, the first and last audiograms were compared. Normal hearing is defined as hearing threshold < 20 dB, mild hearing loss 20 to 40 dB, moderate loss 40 to 70 dB and severe loss is hearing threshold greater than 70 dB, 4F-PTA and HF-PTA were separately assessed. A change from one category to another (using 4F-PTA) was consideredPresence of cochlear enhancement was obtained from reports of 1.8 mm sections, contrast-enhanced FLAIR-MRI through the inner ear. The association between hearing loss progression and age at start of treatment, as well as presence of cochlear enhancement in last MRI was assessed using Fisher’s exact test. Results: Inflammatory markers normalized in 33 (85%) but cochlear enhancement persisted in 16 patients (41%) on treatment. At baseline, 34 (44%) ears had normal hearing on the 4F-PTA, only 28 (36%) ears had normal hearing on HF-PTA (figure 1).
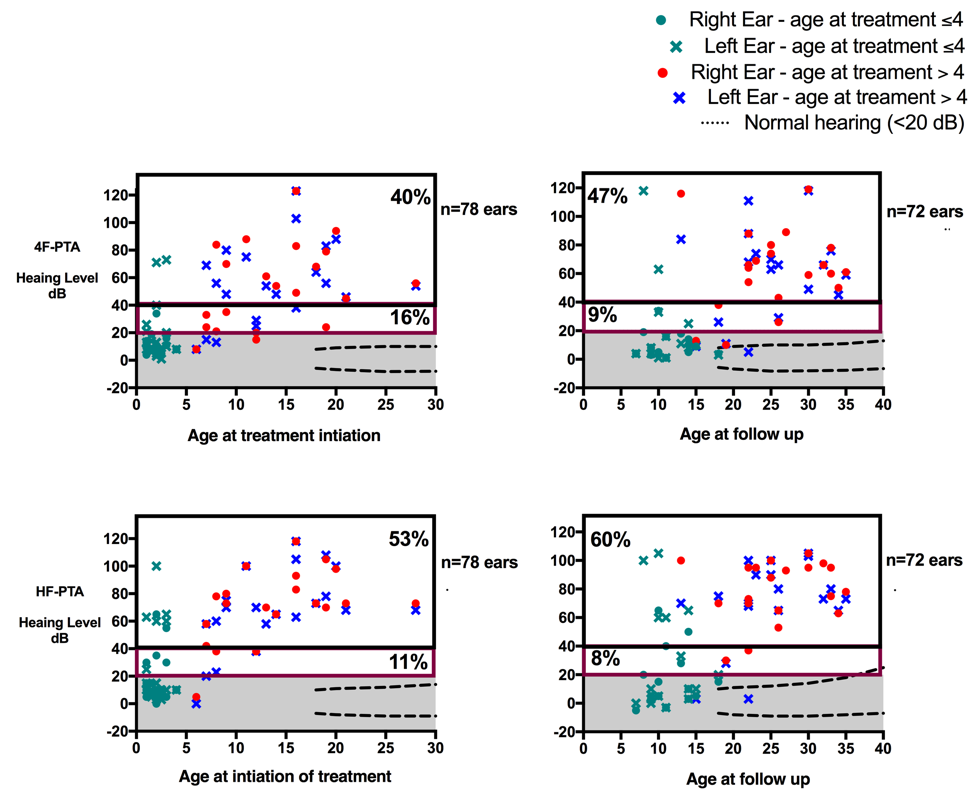
14 patients had normal hearing at baseline (36%); 13 (93%) started treatment at age 4y or younger, eleven of these retained normal hearing at follow up (median follow up 8y, range 4 to 15). Hearing worsened over time in 18 of 56 ears (32%) from 13 patients who had hearing loss at baseline. At last follow up, 2 patients had received cochlear implants for the past 5 and 10 years and 2 years respectively. All patients with progressive hearing loss were treated after age 4 (p = 0.0002). More ears with progressive hearing loss had cochlear enhancement at the time of the last MRI (p = 0.001) (Figure 2).
Conclusion: Progression of hearing loss was prevented in patients starting IL-1 blocking treatment at age of 4 or younger and had normal hearing at baseline. Hearing loss, when present at baseline, typically progressed over time on treatment and was first detected with the HF-PTA. Persistent inner ear enhancement on FLAIR MRI was associated with progressive hearing and suggest the use of FLAIR MRI to monitor inner ear inflammation. Our data points to the need of early treatment in maintaining normal hearing in patients with NOMID.
Reference
Sibley, et al. “Sustained response and prevention of damage progression in NOMID treated with anakinra: three‐and five‐year outcomes.” Arthritis & Rheumatism (2012)
Funding for this study was provided in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
CRP -0-4.99 mg/L-, ESR -0-42 mm/h- *6 patients had disease flare, with high CRP/normal ESR in follow up
Ears with normal, mild and moderate to severe hearing loss at baseline and 10-year follow up are graphed separately based on values of 4F-PTA -500, 1000, 2000, 4000 Hz- -upper panels- and high frequency HF- PTA -6000, 8000 Hz- -lower panels-. Normal hearing is defined as thresholds <20 dB, mild hearing loss is defined as 20 to 40 dB, and moderate to severe hearing loss is defined as hearing thresholds greater than 40 dB. From 14 patients who had normal hearing at baseline in both ears and were treated age 4 or younger, 13 retained hearing in follow up -green color-. Dashed lines denote the 5th and 95th-percentile of age-matched normative thresholds from the International Organization for Standardization ISO 7029. As typically seen in sensorineural hearing loss progression of hearing loss is first seen in the HF-PTA before present in 4FT-PTA.
37 patients had audiogram in follow up: Hearing loss was not observed in patients who started treatment at age <4y who had normal hearing at baseline. Hearing loss progressed in 13 patients who had hearing loss at baseline. All were treated after age 4 -upper panel-. In ears with progressive hearing loss, cochlear enhancement was present in 66.8% of ears compared to 20% of hear that had no progressive hearing loss -p = 0.001-.
To cite this abstract in AMA style:
Alehashemi S, Garg M, King K, Zalewski C, de Jesus A, Butman J, Eisenberg J, Brewer C, KIm J, Goldbach-Mansky R. Preliminary Analysis of Hearing Loss in a Neonatal-Onset Multisystem Inflammatory Disease (NOMID) Cohort Followed over a Mean of 10 Years: Normal Hearing at Baseline and Early Treatment with Anakinra Area Associated with Maintenance of Normal Hearing [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/preliminary-analysis-of-hearing-loss-in-a-neonatal-onset-multisystem-inflammatory-disease-nomid-cohort-followed-over-a-mean-of-10-years-normal-hearing-at-baseline-and-early-treatment-with-anakinra/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/preliminary-analysis-of-hearing-loss-in-a-neonatal-onset-multisystem-inflammatory-disease-nomid-cohort-followed-over-a-mean-of-10-years-normal-hearing-at-baseline-and-early-treatment-with-anakinra/