Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: APS ACTION Registry was created to study the natural course of disease over 10 years in persistently antiphospholipid antibody (aPL) positive patients with or without systemic autoimmune diseases. The objective of this analysis was to describe the new pregnancy outcomes of the aPL-positive patients since the inception of the registry.
Methods: A web-based data capture system is used to store patient demographics, history, and medications. The inclusion criteria are positive aPL according to Updated Sapporo Classification Criteria tested within one year prior to enrollment. Patients are followed every 12±3 months with clinical data and blood collection. We identified patients recorded as “pregnant” during prospective follow-up; new “aPL-related composite pregnancy morbidity” was defined as: a) preterm live birth (PTLB) at or before 37th week due to (pre)eclampsia (PEC) and/or small-for-gestational age (SGA); and b) fetal death (FD) after the 10th week. In addition to descriptive characteristics, we analyzed pregnancy outcomes based on different aPL-related histories.
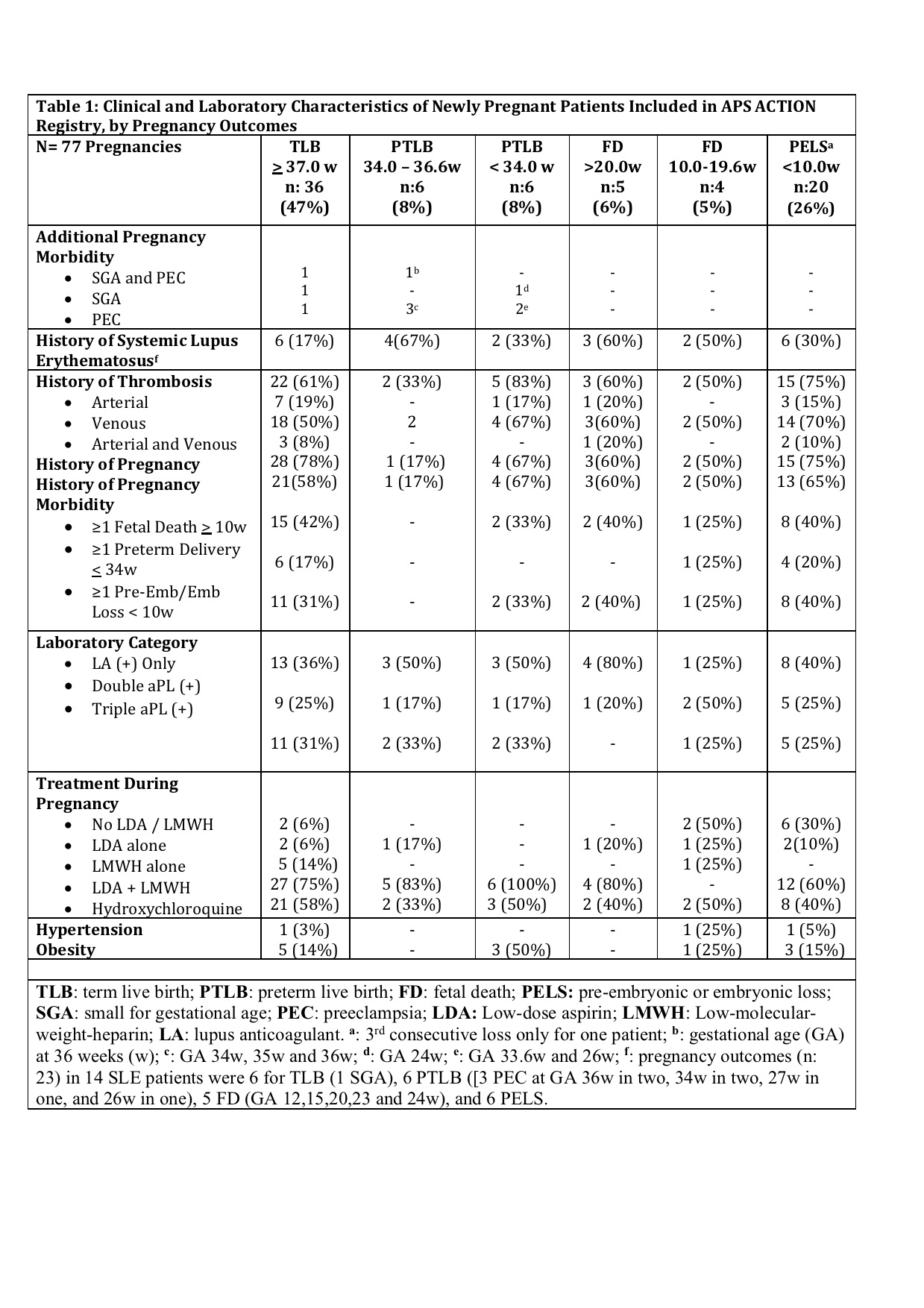
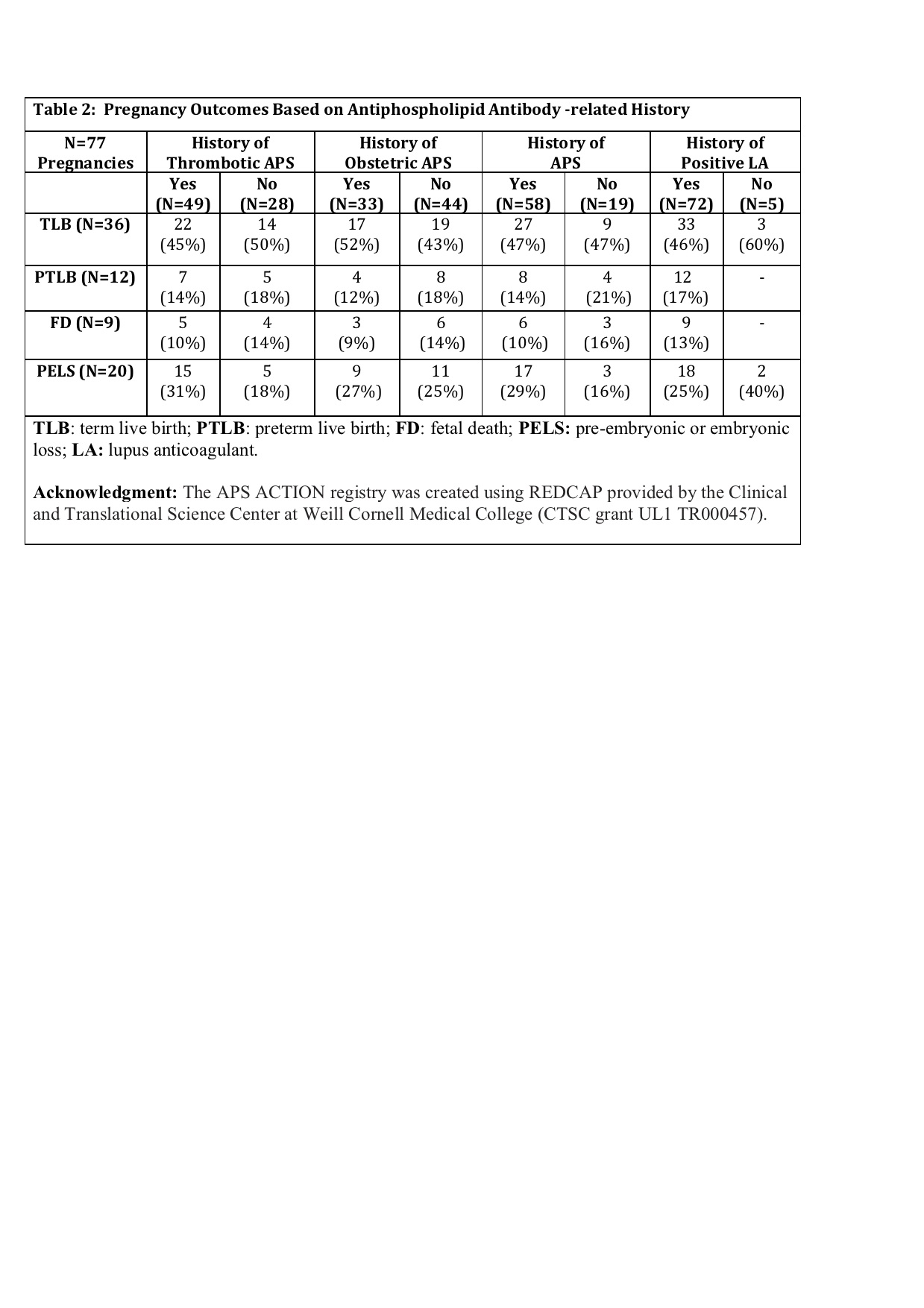
Results: Since the inception of the registry in 2012, 77 completed pregnancies were recorded in 55 patients (mean maternal age: 33.4 ± 5.2y; primary aPL/APS: 41 [75%]; and SLE: 14 [25%]) (Table 1). Of 55 patients, 15 (27%) did not fulfill the clinical APS classification criteria, 8 (15%) had obstetric APS (OAPS) only, 18 (33%) thrombotic APS (TAPS) only, and 14 (25%) both OAPS+TAPS. Pregnancy outcomes are reported in Table 1. Sixty-seven of 77 (87%) pregnancies were treated with low dose aspirin (LDA) and/or low-molecular weight heparin (LMWH) (54 with LDA+LMWH): 9/77 (12%) were due to OAPS only (LDA+LMWH: 7); 21/77 (27%) TAPS only (LDA+LMWH: 18); 21/77 (27%) OAPS and TAPS (LDA+LMWH: 19); and 16/77 (21%) despite no APS classification (LDA+LMWH: 10). Table 2 demonstrates TLB, PTLB, FD, and PELS rates based on the history of thrombotic APS (vs not), obstetric APS (vs not), and APS (vs not); 93% of all patients were lupus anticoagulant [LA] positive (as well as 100% of those who developed aPL-related composite pregnancy morbidity).
Conclusion: In our multi-center international prospective aPL-positive cohort, of 77 pregnancies in 55 patients observed prospectively, 20 (26%) were complicated by (pre)embryonic losses. Of the remaining 57 pregnancies, aPL-related composite pregnancy morbidity was observed in 16/57 (28%) of pregnancies including 7/57 (12%) pre-term live births with SGA and/or PEC, and 9/57 (16%) fetal deaths.
To cite this abstract in AMA style:
Erton Z, Sevim E, de Jesus G, Cervera R, Ji L, Pengo V, Ugarte A, Andrade D, Andreoli L, Atsumi T, Fortin P, Gerosa M, Zuo Y, Petri M, Sciascia S, Tektonidou M, Aguirre M, Branch D, Erkan D, APS ACTION o. Pregnancy Outcomes of Antiphospholipid Antibody Positive Patients: Prospective Results from AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/pregnancy-outcomes-of-antiphospholipid-antibody-positive-patients-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-da-2/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-outcomes-of-antiphospholipid-antibody-positive-patients-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-clinical-da-2/