Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Data pertaining to the use of biologics in immunologic diseases are limited on their use during pregnancy. Guselkumab (GUS) is a human IgG1λ mAb approved for moderate-to-severe psoriasis (PsO) and active PsA; it is currently in clinical development for IBD. IgG1λ antibodies are known to cross the placental barrier and have the potential to affect pregnancy outcomes. We aimed to assess pregnancy outcomes in pregnant women exposed to GUS.
Methods: Cumulative data from the Janssen Global Safety Database reported through 12 July 2023 are summarized descriptively for medically confirmed and unconfirmed pregnancies with maternal exposure to GUS before conception (within 3 months prior to confirmed pregnancy), during the first trimester (T1), after the first trimester (T2, T3), or any time during confirmed pregnancy. Pregnancy outcomes were classified as live births with or without congenital anomalies; spontaneous abortions; elective terminations, stillbirths, or unspecified abortions with or without fetal defects; fetal deaths; ectopic pregnancies; induced/missed abortions; or pregnancies that are ongoing or that have no reported outcome.
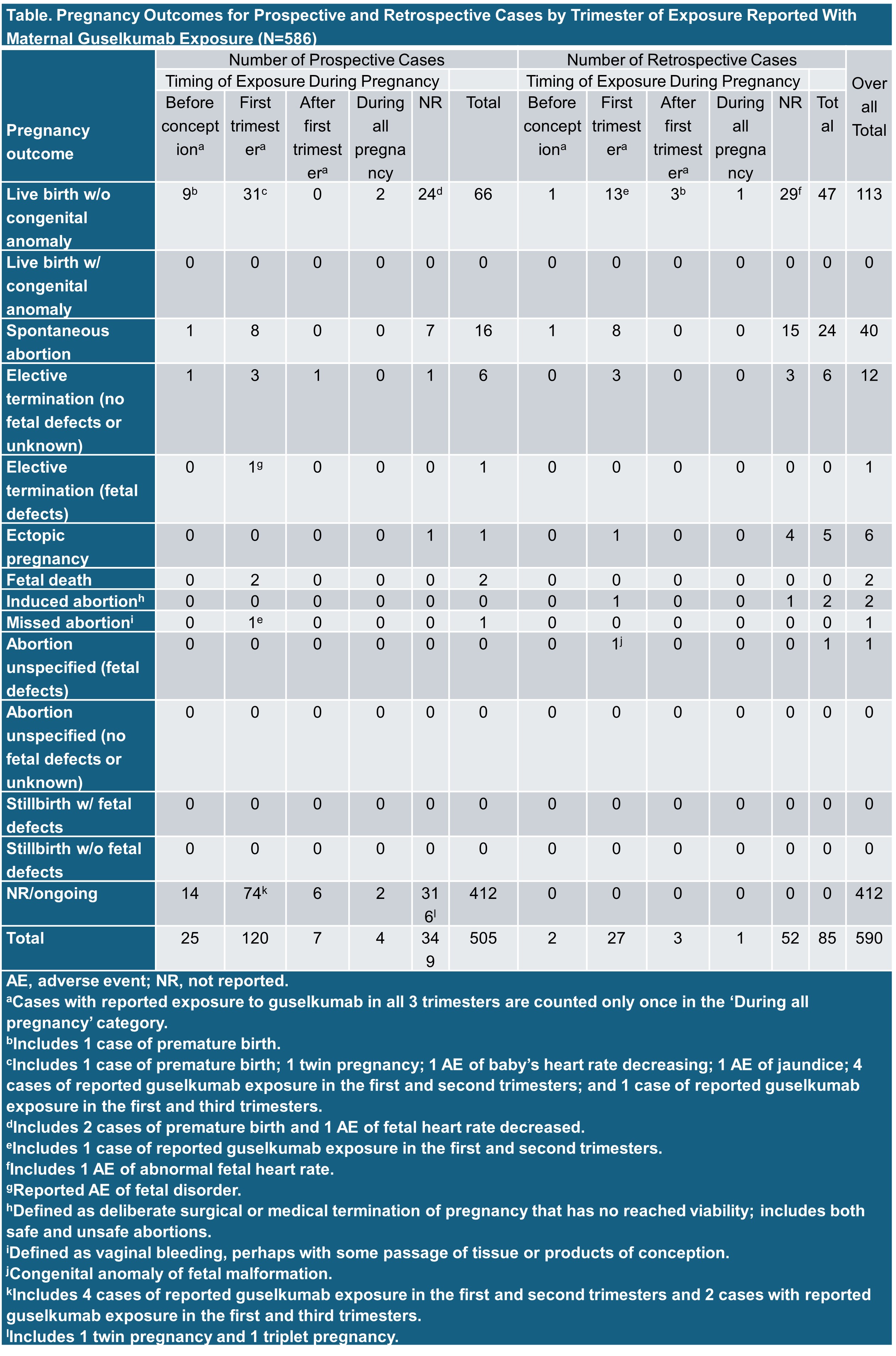
Results: Overall, 590 pregnancy events (twins=2; triplets=1) in 586 women exposed to GUS have been reported prospectively (N=505) or retrospectively (N=85) (Table). When indication was reported (N=412), GUS was administered for PsO (90.0% [371/412]); PsA (2.2% [9/412]), both PsO and PsA (3.4% [14/412]), and IBD (1.5% [6/412]). Of the pregnancies reporting guselkumab trimester exposure (N=189), exposure to GUS occurred before conception (14.3% [27/189]), during T1 (77.8% [147/189]), during T2 or T3 (5.3% [10/189]), or any time during pregnancy (2.6% [5/189]). Mean duration of GUS exposure prior to pregnancy in known cases (N=158) was 385 days (median, 238 [0–1717 days]). Where reported (N=349), mean maternal age was 32.0 years (median, 32 [19–56 years]). Outcomes were reported for 30.2% (178/590) of pregnancy events, which included live birth (63.5% [113/178]), spontaneous abortion (22.5% [40/178]), elective termination (no fetal defects or unknown) (6.7% [12/178]), ectopic pregnancy (3.4% [6/178]), induced abortion (1.1% [2/178]), fetal death (1.1% [2/178]), and 1 event each (0.6%) of elective termination with a fetal defect, missed abortion, and unspecified abortion with fetal defects.
Conclusion: In pregnancy cases with known outcomes, rates of live births, spontaneous and elective/induced abortions, and congenital anomalies in women exposed to GUS ≤3 months before or during pregnancy were consistent with rates reported for the United States general population.1,2 These results are limited by missing data on pregnancy outcomes, maternal age statistics, concomitant medication use, other risk factors, and duration of GUS exposure. These findings represent a robust analysis of maternal GUS-exposed pregnancy outcomes to date, however, additional evidence is needed to increase understanding of the effects of GUS exposure on pregnancy outcomes across disease indications.
1. Centers for Disease Control and Prevention, https://www.cdc.gov/ncbddd/birthdefects/data.html
2. Griebel CP, et al. Am Fam Physician. 2005;72:1243-50
To cite this abstract in AMA style:
Lin C, Geldhof A, Ballina M, Patel H, Li H. Pregnancy Outcomes in Women Exposed to Guselkumab: Review of Cases Reported to the Manufacturer’s Global Safety Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/pregnancy-outcomes-in-women-exposed-to-guselkumab-review-of-cases-reported-to-the-manufacturers-global-safety-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-outcomes-in-women-exposed-to-guselkumab-review-of-cases-reported-to-the-manufacturers-global-safety-database/