Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Women are affected by RA 3–4 times more often than men. RA treatment choice during pregnancy can be a challenge for women to minimize fetal toxicity while maintaining disease control. The new FDA ‘Pregnancy and Lactation Labeling Rule’ removed pregnancy categories requiring product labels to be updated.1 Given limited information, it is important to continually assess available data on RA therapy use during pregnancy.
Methods: All pregnancy cases reported to the manufacturer were re-coded based on ICH E2B (R2) Guideline2 and MedDRA Points to Consider.3 A comprehensive, post-coding change evaluation of pregnancy cases from available sources was performed cumulatively up to March 31, 2016.
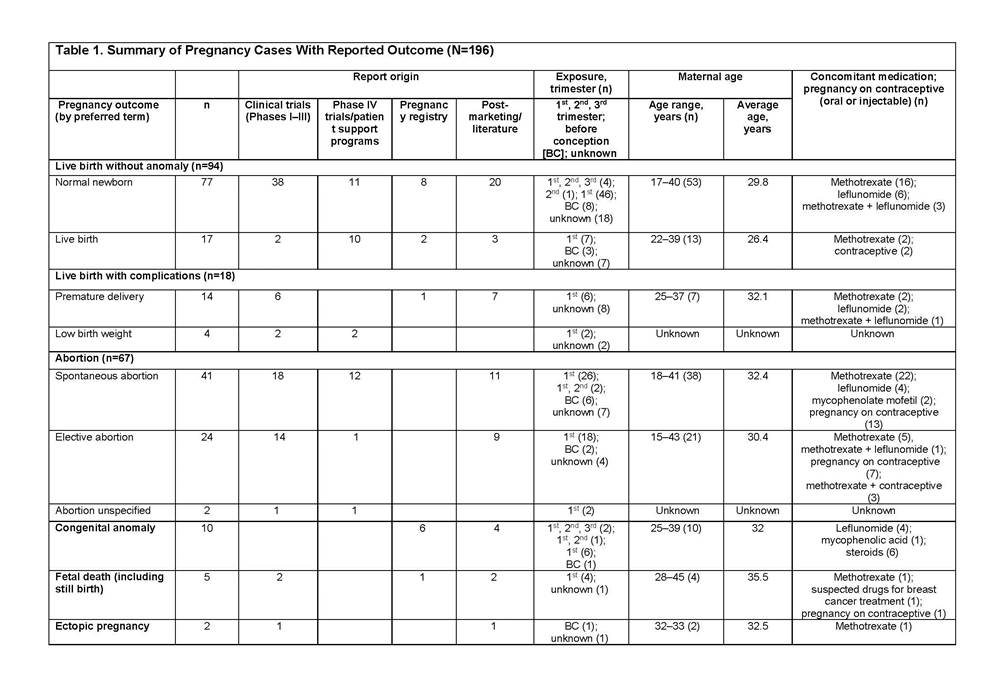
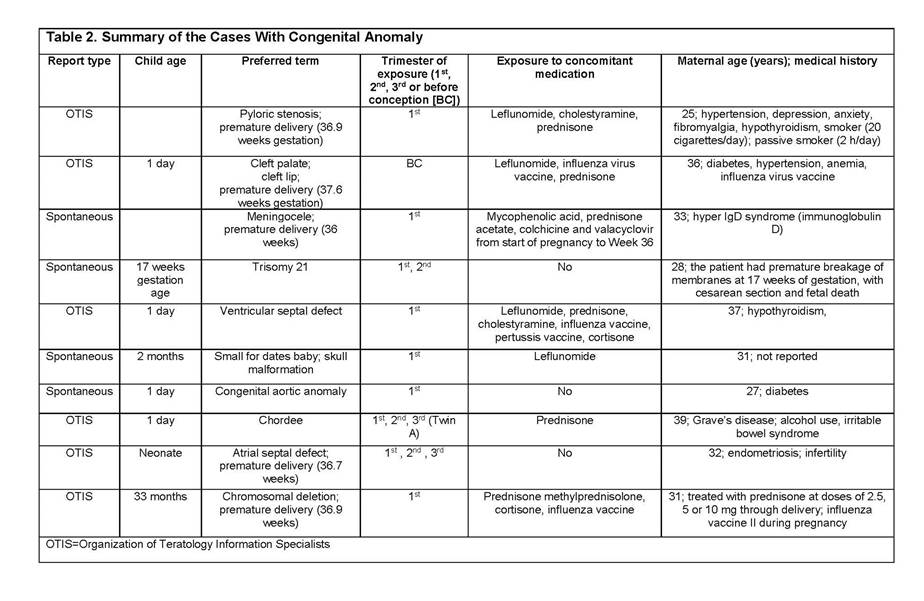
Results: In total, 356 cases were reported: clinical trials (114), spontaneous/literature (133), patient support programs/Phase IV trials (89), and Organization of Teratology Information Specialists Pregnancy Register (20). Most abatacept exposure was limited to the first trimester (118). Outcomes were reported in 196 cases (Table 1): 48% normal newborn/live births (4 full-term abatacept exposure cases), 21% spontaneous abortions, 9% live births with complications including premature delivery and low birth weight. There were 10 congenital anomalies in patients with multiple risk factors4 (2 full-term abatacept exposure cases, Table 2).
Conclusion: As shown, reports of spontaneous abortion, premature delivery, and low birth weight among patients treated with abatacept are low and consistent with the literature.5–8 The available data do not suggest an increased risk of adverse pregnancy outcomes with abatacept in the indicated population. However, abatacept should be used during pregnancy only if the benefit to the mother justifies potential risk to the fetus.9 The outcomes of abatacept-exposed pregnancies will continue to be monitored via pharmacovigilance and the Pregnancy Registry.
To cite this abstract in AMA style:
Yu H, Angelini K, Dominique A, Simon T. Pregnancy Outcomes in Patients with Rheumatoid Arthritis Treated with Abatacept – Review of a Safety Database [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/pregnancy-outcomes-in-patients-with-rheumatoid-arthritis-treated-with-abatacept-review-of-a-safety-database/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-outcomes-in-patients-with-rheumatoid-arthritis-treated-with-abatacept-review-of-a-safety-database/