Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic lupus erythematosus (SLE) predominantly affects females of reproductive age. Cyclophosphamide, an alkylating agent, labelled category D for pregnancy, is used for induction in treatment of severe manifestations of lupus. Previous studies have shown increased rate of primary ovarian insufficiency causing infertility in patients who have received cyclophosphamide. But recent studies with cancer treatment suggest fair pregnancy outcomes in such patients. Now, with an earlier age at lupus diagnosis, increasing maternal age for pregnancy, and with treatment protocols using lower doses of medication, we have an increasing population of lupus patients who plan to conceive after having received cyclophosphamide. The objective of our study was to add to the available data in identifying the impact of cyclophosphamide exposure on fertility and pregnancy outcomes.
Methods: Using diagnostic codes, we searched for female patients fulfilling ACR criteria for SLE who received cyclophosphamide in the time period from 2000-2018 in our academic institution. We have identified about 400 such patients and as a pilot project of an ongoing study, we performed a retrospective chart review on 100 of those patients. Each patient was evaluated for demographic and serological profile, age of patient at the time of cyclophosphamide infusion and pregnancy and subsequent maternal and fetal outcomes or infertility. We compared these variables using two sample T-test. This is part of an ongoing study and more patients are expected to be added to our final analysis.
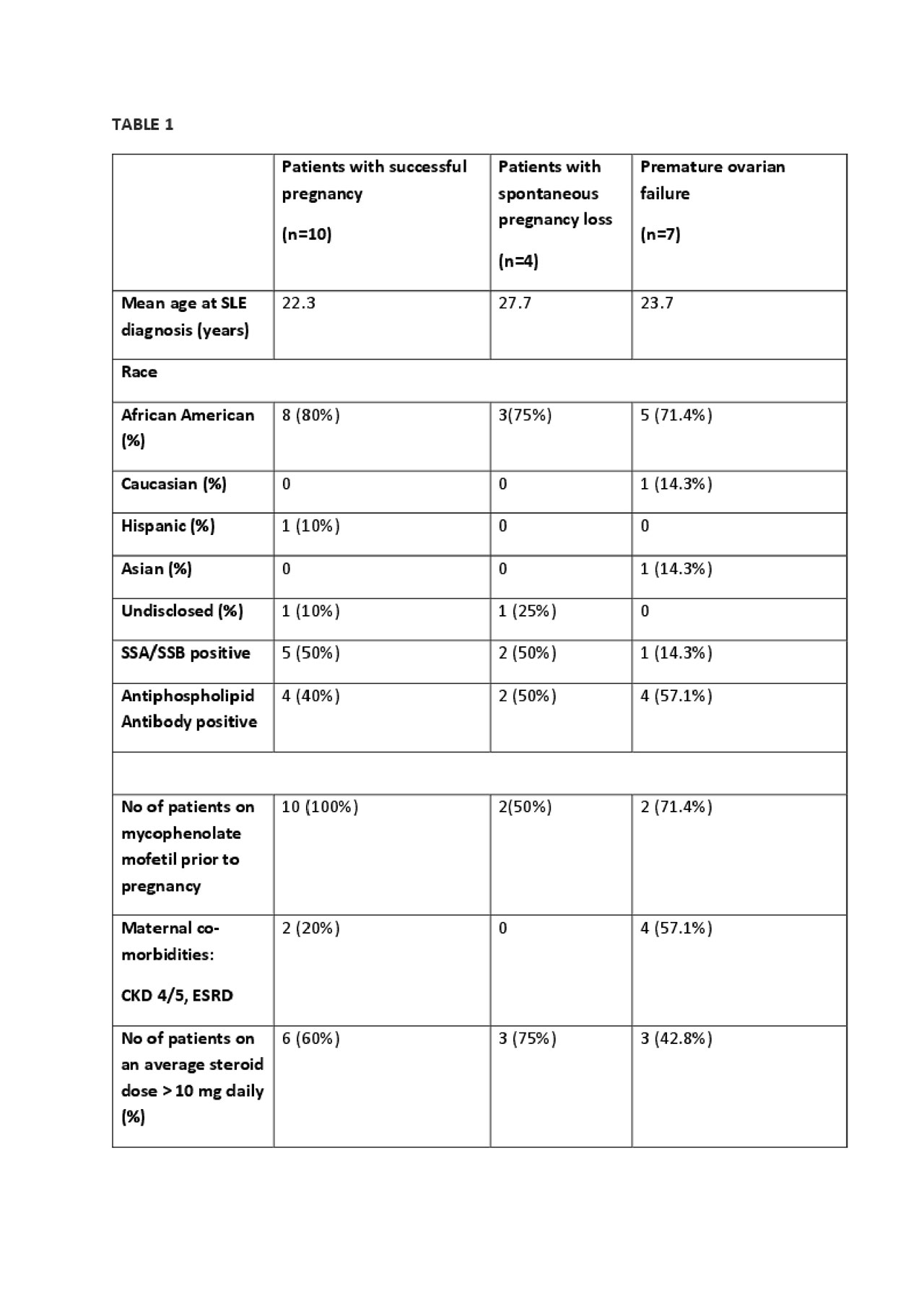
Results: Of the 100 female SLE patients reviewed in this study, 82 were given cyclophosphamide for biopsy proven nephritis, 12 for cerebritis, 3 for vasculitis and 1 each for diffuse alveolar hemorrhage and severe cutaneous disease. Fertility counselling was documented in 54% of the patients at the time of medication initiation. 19 patients had subsequent pregnancies; 10 with healthy maternal and fetal outcomes, 5 underwent elective termination within first trimester and 4 had spontaneous miscarriages. Only 7 out of 100 females were identified to have premature ovarian failure which included 4 who also had end stage renal disease. (Table 1)
Average age (in years) at the time of cyclophosphamide administration was 24.9 in patients with successful pregnancy; 33.4 with spontaneous miscarriage and 32 with premature ovarian failure. Average duration (in months) between the last cyclophosphamide infusion and subsequent pregnancy was 53.5 in patients with successful pregnancy vs 19.3 in patients with a spontaneous miscarriage (p 0.144). Average cumulative dose of cyclophosphamide (in mg) was 3733 in patients with successful pregnancy compared to 2000 with spontaneous miscarriage (p 0.1529) and 4375 with ovarian insufficiency (p 0.0403).
Conclusion: Our findings suggest that a longer time interval between the last cyclophosphamide infusion and pregnancy was more favorable for a successful pregnancy outcome. However, a higher cumulative dose is more likely to be associated with premature ovarian failure.
To cite this abstract in AMA style:
Sen M, Kurl A, Vashi T, Khosroshahi A. Pregnancy in Patients with Systemic Lupus Erythematosus After Cyclophosphamide Therapy [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/pregnancy-in-patients-with-systemic-lupus-erythematosus-after-cyclophosphamide-therapy/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/pregnancy-in-patients-with-systemic-lupus-erythematosus-after-cyclophosphamide-therapy/