Session Information
Date: Monday, November 18, 2024
Title: SpA Including PsA – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
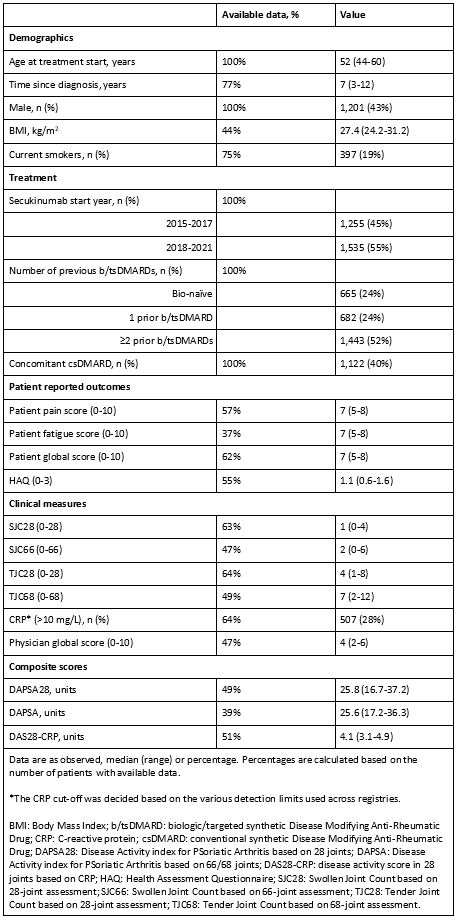
Background/Purpose: Several predictors of treatment response to tumour necrosis factor inhibitors (TNFi) in routine care have been reported in psoriatic arthritis (PsA). However, data on predictors of treatment response to secukinumab, an interleukin-17A inhibitor, remain sparse. In patients with PsA initiating secukinumab in clinical practice, we therefore aimed to identify baseline predictors of achieving Disease Activity index for PsA in 28 joints (DAPSA28) low disease activity (LDA), and DAPSA28 moderate response at 6 months, as well as treatment continuation at 12 months.
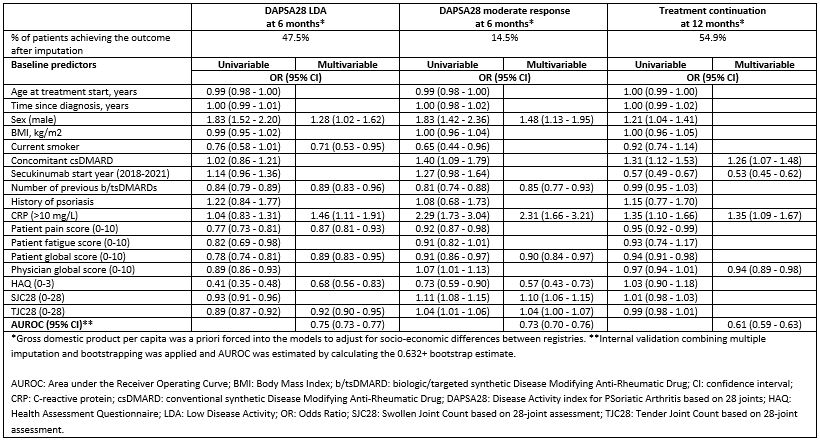
Methods: Data on patients with PsA, initiating a first secukinumab treatment between 2015 and 2021, were pooled and analyzed from 14 registries participating in the European Spondyloarthritis (EuroSpA) collaboration. Logistic regression analyses were performed to identify baseline (-/+30 days from treatment start) predictors of DAPSA28 LDA (≤14) and DAPSA28 moderate response (75% improvement from the baseline DAPSA28) at 6 months, and treatment continuation at 12 months. We explored 17 baseline demographic and clinical characteristics as potential predictors. Multiple imputation by chained equations was applied to impute missing baseline variables and DAPSA28 at 6 months to account for attrition. For each outcome, variables were identified as predictors by applying backward selection on multiply imputed data (30 imputed datasets). The final model was fitted to all imputed datasets and the model estimates were pooled. The performance of the final multivariable models was assessed by the Area under the Receiver Operating Curve (AUROC).
Results: We included 2,790 patients, of whom 1,201 (43%) were male. Baseline patient characteristics [median (range)] were: age of 52 (44-60) years, time since diagnosis of 7 (3-12) years and DAPSA28 of 25.8 (16.7-37.2) (Table 1). Among the patients, 24%/24%/52% had previously received 0/1/≥2 biologic or targeted synthetic disease modifying anti-rheumatic drugs (b/tsDMARDs), respectively. Eight predictors were identified for DAPSA28 LDA, seven for DAPSA28 moderate response and four for treatment continuation (Table 2). Elevated C-reactive protein ( >10 mg/L vs. ≤10 mg/L) increased the odds of achieving all three outcomes and was the only common predictor. Additional positive common predictors of achieving DAPSA28 LDA and DAPSA28 moderate response were: male sex, fewer previous b/tsDMARDs, lower patient global score, and lower score in health assessment questionnaire, while 28-tender joint count showed inconsistent results across outcomes. The model performance was good for DAPSA28 LDA and DAPSA28 moderate response (AUROC between 0.7 and 0.8) and moderate for treatment continuation (AUROC between 0.6 and 0.7).
Conclusion: In a large European cohort of patients with PsA initiating secukinumab, baseline predictors of LDA, treatment response and continuation were identified. Our findings are in line with previous studies exploring responses to TNFi treatment, suggesting that, regardless of the treatment mode of action, patient-related factors are important for the understanding of real-world drug effectiveness.
To cite this abstract in AMA style:
Georgiadis S, Ostergaard M, Heberg J, Ahmadzay Z, Michelsen B, Rasmussen S, Kazemi M, Karlsson Wallman J, Olofsson T, Glintborg B, Loft A, Castrejón I, Šenolt L, Nissen M, Moeller B, Garcia J, Barcelos F, Rotar Z, Perdan-Pikmajer K, Codreanu C, Mogosan C, Laas K, Vorobjov S, Gudbjornsson B, Gröndal G, Nordstrom D, Hokkanen A, Mielnik P, Kvien T, Kenar G, Van De Sande M, Hetland M, Oernbjerg L. Predictors of Treatment Response and Continuation in Patients with Psoriatic Arthritis Initiating Secukinumab – Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/predictors-of-treatment-response-and-continuation-in-patients-with-psoriatic-arthritis-initiating-secukinumab-results-from-the-eurospa-collaboration/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-treatment-response-and-continuation-in-patients-with-psoriatic-arthritis-initiating-secukinumab-results-from-the-eurospa-collaboration/