Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Biologics therapy survival is often used as a proxy for treatment effectiveness and safety. However, it may be influenced by patient characteristics, utilization patterns, and available treatment alternatives, which tend to change over time. The objective of this analysis was to assess in Canadian routine clinical practice the survival of treatment with adalimumab (ADA) in AS and PsA, and the determinants of ADA survival.
Methods: COMPLETE-AS is an ongoing Canadian observational study of anti-TNFα naïve adults with active AS or PsA who require, per the judgment of the treating physician, change in current treatment. Patients are followed for up to 2 years. In the current analysis, patients initiating ADA were included. Kaplan Meier (KM) estimates and Cox proportional models were used in the analysis. Potential predictors evaluated were age, gender, enrollment period, combination treatment with non-biologic DMARD(s) (nbDMARDs) vs. monotherapy, baseline disease activity (AS: BASDAI; PsA: DAS28), and baseline functional activity (AS: BASFI; PsA: HAQ). In a secondary analysis, the achievement of BASDAI 50 (AS) and ΔDAS28≥1.2 (PsA) at 3 months were also considered.
Results: A total of 459 AS and 278 PsA patients were included in the analysis. Mean age of the AS and PsA patient cohorts was 43.9 and 52.0 years, respectively. Mean BASDAI and DAS28 scores in AS and PsA patients were 6.4 and 4.8, respectively. KM-based mean (95% CI) time to ADA discontinuation was 1.77 (1.72-1.82) years and 1.83 (1.77-1.88) years for AS and PsA patients, respectively.
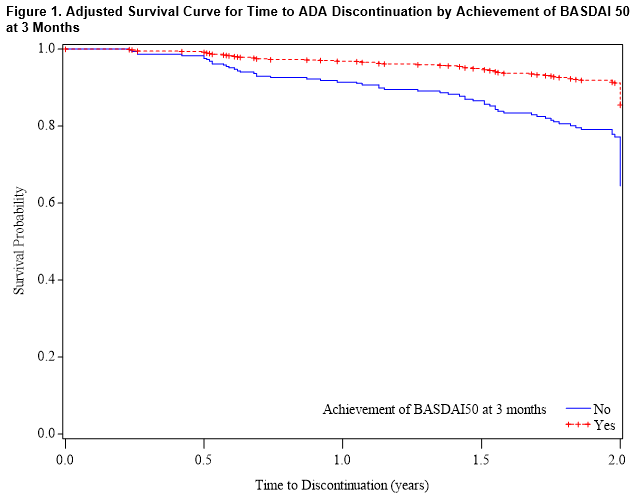
Among AS patients, BASFI score at baseline was identified as the only significant predictor of ADA survival, (HR [95% CI]: 1.17 [1.08-1.28]). In the secondary analysis considering response to treatment at 3 months, achievement of BASDAI 50 was associated with significantly lower hazard for discontinuation (0.36 [0.20-0.65]; Figure 1); in this analysis, age (0.98 [0.97-1.00]) and baseline BASFI score (1.24 [1.11-1.39]) were also significant predictors of ADA retention.
Among PsA patients, male gender was identified as the only significant (positive) predictor of ADA survival, both in the primary (0.50 [0.29-0.85]) and secondary (0.39 [0.18-0.85]) analysis.
Conclusion: The results of this analysis have shown that early achievement of BASDAI 50 is associated with improved long-term retention of ADA treatment among AS patients. Furthermore, gender differences may exist in the real-world survival of ADA treatment in PsA.
To cite this abstract in AMA style:
Bessette L, Khraishi M, Stewart J, Chow A, Pavlova V, Florica B, Remple VP. Predictors of Survival of Adalimumab Treatment in the Management of Ankylosing Spondylitis and Psoriatic Arthritis in Canadian Routine Care [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/predictors-of-survival-of-adalimumab-treatment-in-the-management-of-ankylosing-spondylitis-and-psoriatic-arthritis-in-canadian-routine-care/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-survival-of-adalimumab-treatment-in-the-management-of-ankylosing-spondylitis-and-psoriatic-arthritis-in-canadian-routine-care/