Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Identification of predictive clinical factors of long-term treatment response in non-radiographic axial spondyloarthritis (nr-axSpA) may contribute to improved management of patients with this chronic disease. Certolizumab pegol (CZP) is currently the only FDA-approved tumor necrosis factor inhibitor (TNFi) for treatment of nr‑axSpA.1 We aim to identify whether any demographic or baseline characteristics of nr-axSpA patients from the C-axSpAnd study2 are predictive of achieving a clinical response after 1 year of CZP treatment.
Methods: C-axSpAnd (NCT02552212) is a phase 3, interventional multicenter study including a completed 52‑week double-blind, placebo-controlled period. Full study design is reported elsewhere.2 Multivariate stepwise logistic regression analysis was used to identify predictors of response for the primary efficacy variable (ASDAS – major improvement [ASDAS-MI] at Week 52) and the main secondary efficacy variable (ASAS40 at Week 52) in patients randomized to CZP 200 mg every 2 weeks (Q2W). Predictive factors used in the model included demographic and baseline characteristics, and clinical outcomes at Week 12. A p value ≤0.05 was required for forward selection into the model and p=0.1 for backward elimination from the model. Non-responder imputation was used to account for missing data or values collected after switching to open-label treatment. A sensitivity analysis was conducted to account for patients who had changes in their non-biologic background medication during the 52-week placebo-controlled phase.
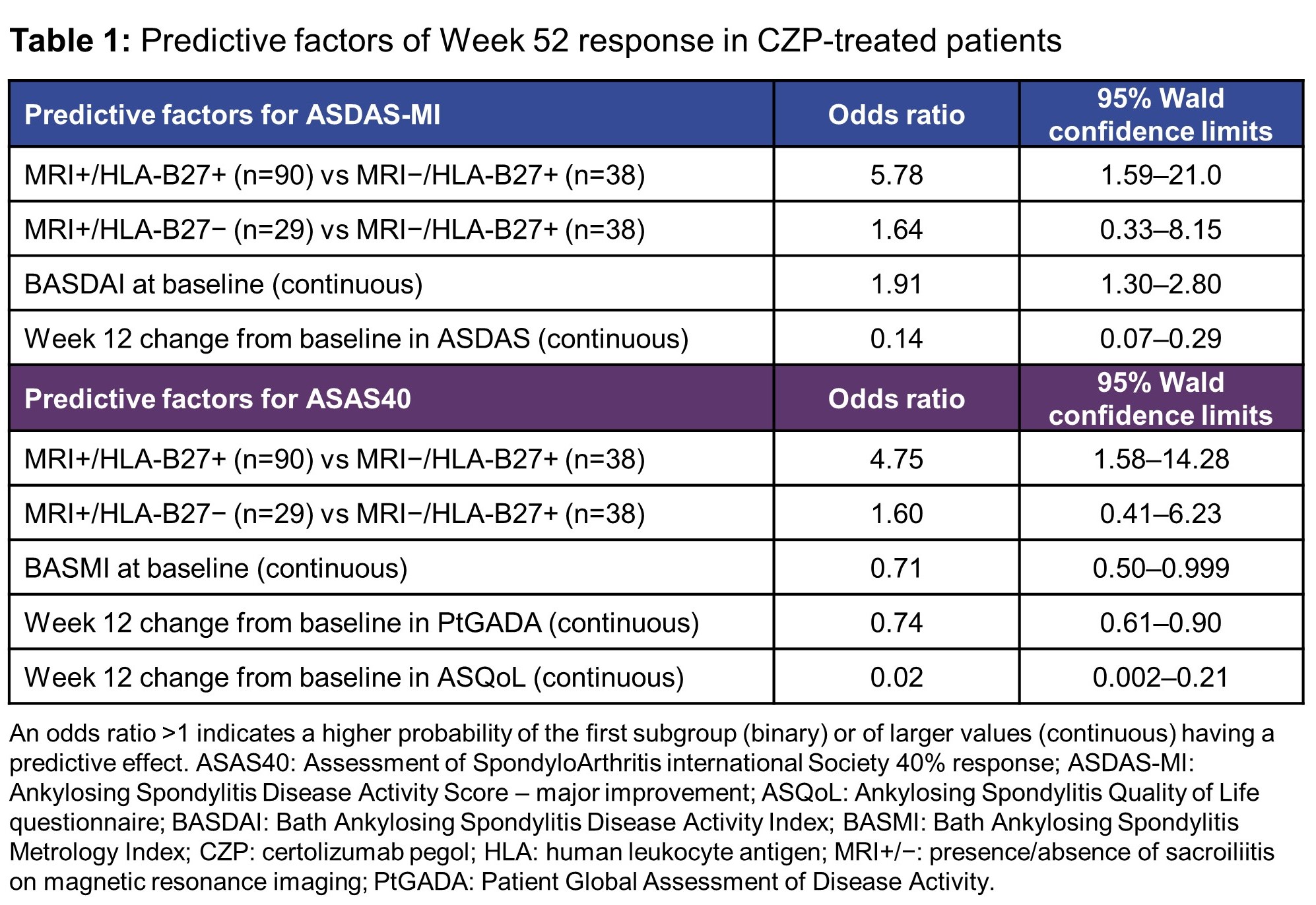
Results: 159/317 patients were randomized to CZP 200 mg Q2W and 158/317 to placebo. Predictive factors identified for Week 52 ASDAS-MI in the CZP-treated patients included those who were positive for both presence of sacroiliitis on MRI (MRI+) and human leukocyte antigen (HLA)-B27 (HLA+), those with a higher BASDAI at baseline, and those with a larger Week 12 improvement in ASDAS (Table 1). For ASAS40 response, MRI+/HLA‑B27+ was also identified as a predictor of Week 52 response, along with a lower baseline BASMI and larger Week 12 improvements in PtGADA and ASQoL and (Table 1). Sensitivity analysis identified the same predictors for ASDAS-MI and ASAS40, with the exception of change from baseline in PtGADA as a predictor of ASAS40. Sensitivity analysis also identified achievement of Week 12 ASAS40 as a predictor of Week 52 ASAS40. In placebo-treated patients, no meaningful predictors of response at Week 52 were identified.
Conclusion: Presence of sacroiliitis on MRI and HLA-B27 positivity were identified as consistent predictors of Week 52 response (ASDAS-MI and ASAS40) in nr‑axSpA patients treated with CZP. To our knowledge, this is the first report from an interventional 52-week placebo-controlled study in nr‑axSpA to identify objective clinical features, particularly the presence of sacroiliac joint inflammation, as being predictive of response.
References: 1. Ashrafi M. Curr Opin Rheumatol 2020;32:321–9; 2. Deodhar A. Arthritis Rheumatol 2019;71:1101–11.
To cite this abstract in AMA style:
Maksymowych W, Kumke T, Auteri S, Hoepken B, Bauer L, Rudwaleit M. Predictors of Response in Patients with Non-Radiographic Axial Spondyloarthritis Receiving Certolizumab Pegol in the C-axSpAnd Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/predictors-of-response-in-patients-with-non-radiographic-axial-spondyloarthritis-receiving-certolizumab-pegol-in-the-c-axspand-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-response-in-patients-with-non-radiographic-axial-spondyloarthritis-receiving-certolizumab-pegol-in-the-c-axspand-study/