Session Information
Date: Monday, November 9, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment III: Axial Spondyloarthritis (2028–2032)
Session Type: Abstract Session
Session Time: 4:00PM-4:50PM
Background/Purpose: RE-EMBARK was a phase 4, multicenter, open-label, 3-period study that evaluated etanercept (ETN) withdrawal and retreatment in adult patients with non-radiographic axial spondyloarthritis (nr-axSpA) and an adequate response to initial ETN treatment. The aim of the current analyses was to explore baseline predictors of the maintenance and regain of inactive disease during Period 2 and Period 3 of RE-EMBARK, respectively.
Methods: RE-EMBARK enrolled patients aged 18 to < 50 years with active nr-axSpA, an inadequate response to ≥2 non-steroidal anti-inflammatory drugs (NSAIDs), and on a stable dose of 1 NSAID for ≥2 weeks prior to first study dose of ETN. Active nr-axSpA was defined as the combination of meeting Assessment in SpondyloArthritis international Society (ASAS) criteria, having active symptoms of nr-axSpA, and having an Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP) ≥2.1. In Period 1, all patients received ETN (50 mg once weekly) and background NSAID for 24 weeks. Patients who achieved inactive disease (ASDAS-CRP < 1.3) at Week 24 were eligible to enter Period 2. In Period 2, ETN was withdrawn (without downward tapering) for up to 40 weeks while background NSAID was maintained. Patients who experienced a flare (ASDAS with erythrocyte sedimentation rate ≥2.1) during Period 2 were eligible to immediately enter Period 3 in which they were retreated with ETN (50 mg once weekly) for 12 weeks. Baseline characteristics of the patients who entered Period 2 and Period 3 (2 separate baselines) were analyzed post hoc as categorical predictors of the maintenance of inactive disease and the regain of inactive disease, respectively, using univariate logistic regression models and stepwise multivariate logistic regression models. Analyses were based on observed cases.
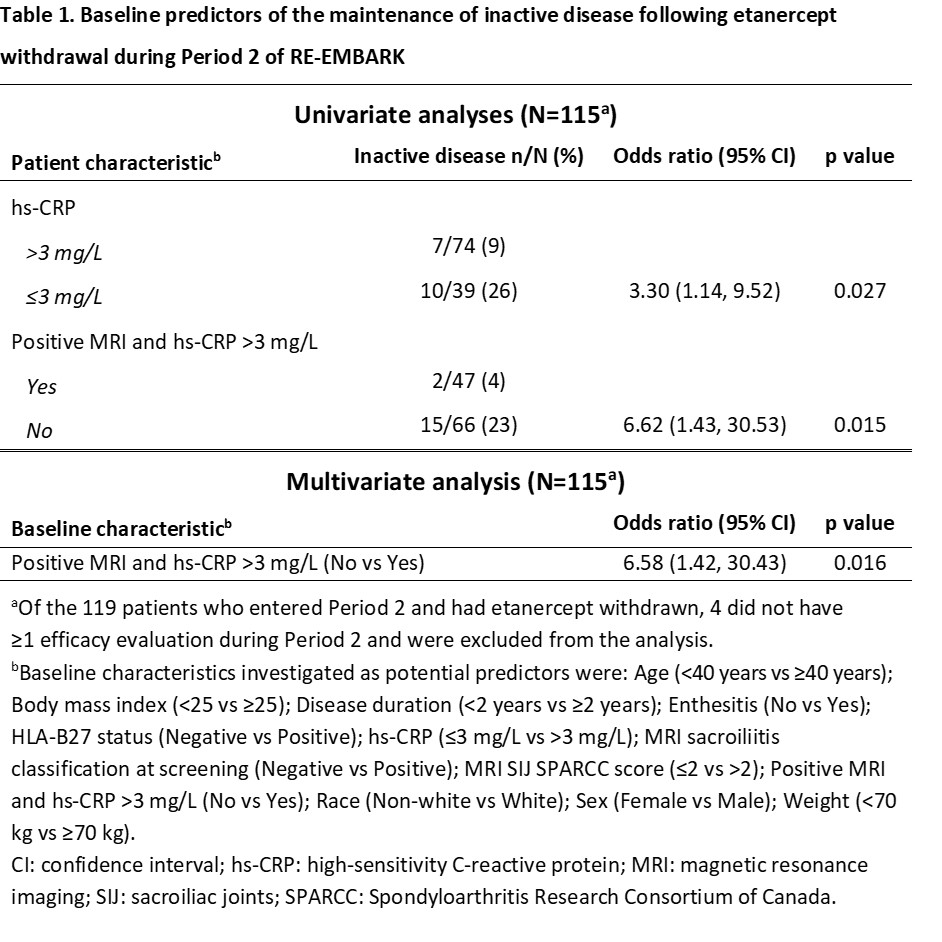
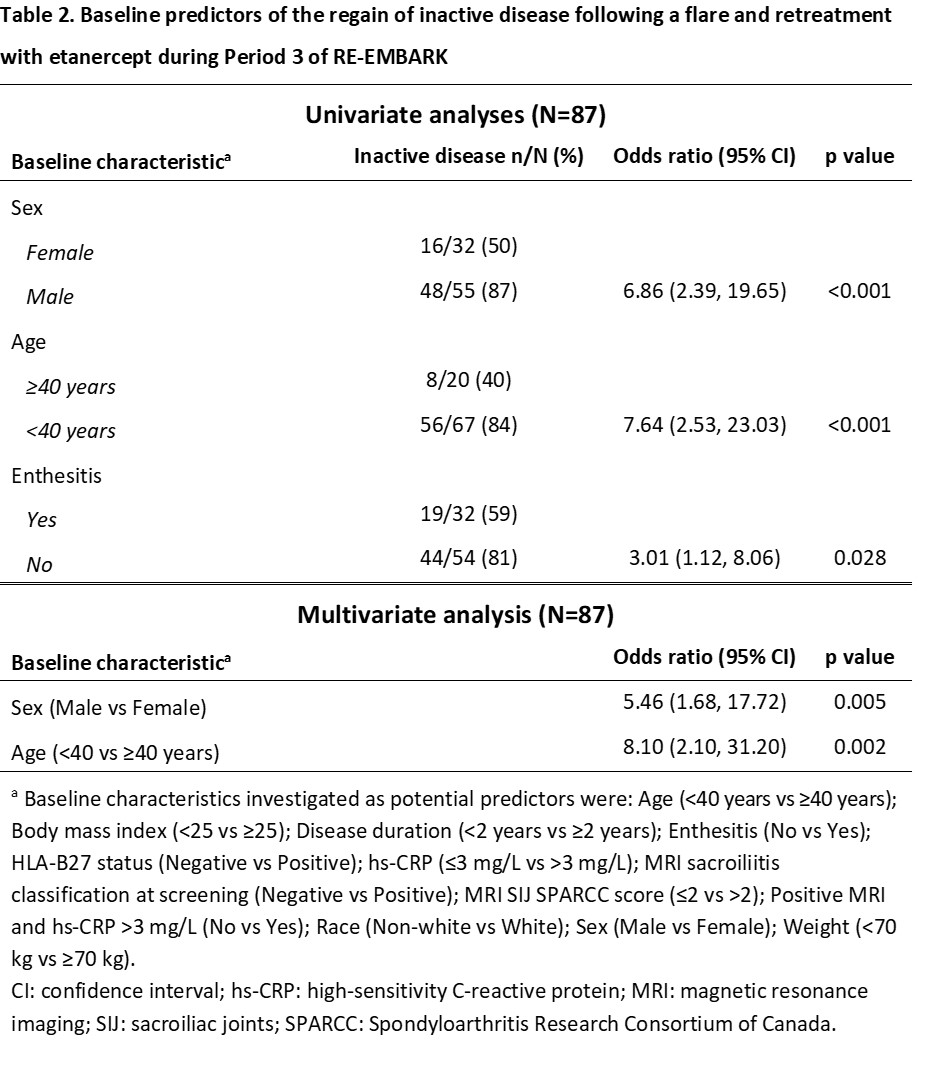
Results: A total of 209 patients were treated during Period 1 and 119 (57%) achieved inactive disease at Week 24 and had ETN withdrawn after entering Period 2. Univariate analyses showed that high-sensitivity CRP (hs-CRP) ≤3 mg/L and the absence of a combination of positive MRI and hs-CRP >3 mg/L were associated with the maintenance of inactive disease following ETN withdrawal (Table 1). Multivariate analysis showed that the absence of a combination of positive MRI and hs-CRP >3 mg/L was a significant predictor of maintaining inactive disease following ETN withdrawal. There were 87 (73%) of the 119 patients in Period 2 who experienced a flare following ETN withdrawal and were retreated during Period 3. Univariate analyses showed that male sex, age < 40 years, and the absence of enthesitis were associated with the regain of inactive disease following retreatment, with multivariate analysis showing that male sex and age < 40 years were significant predictors (Table 2).
Conclusion:
The absence of a combination of positive MRI and hs-CRP >3mg/L at baseline was a significant predictor of adult patients with nr-axSpA maintaining inactive disease following withdrawal of ETN over 40 weeks. Male sex and age < 40 years were significant predictors of a patient regaining inactive disease after a flare and subsequent 12-week retreatment with ETN.
To cite this abstract in AMA style:
Van den Bosch F, Wei J, Blanco F, Selema P, Graham D, Arthur E, Tsekouras V, Vlahos B, Zang C, Deodhar A, Nash P. Predictors of Maintaining Inactive Disease After Etanercept Withdrawal, and Regaining Inactive Disease Status After Flare and Retreatment, in Adults with Non-radiographic Axial Spondyloarthritis: Results from RE-EMBARK [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/predictors-of-maintaining-inactive-disease-after-etanercept-withdrawal-and-regaining-inactive-disease-status-after-flare-and-retreatment-in-adults-with-non-radiographic-axial-spondyloarthritis-resu/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-maintaining-inactive-disease-after-etanercept-withdrawal-and-regaining-inactive-disease-status-after-flare-and-retreatment-in-adults-with-non-radiographic-axial-spondyloarthritis-resu/