Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: To assess baseline demographics and clinical characteristics of patients with PsA that are most associated with achievement of clinical response to intravenous (IV) secukinumab in the phase 3 INVIGORATE-2 study.
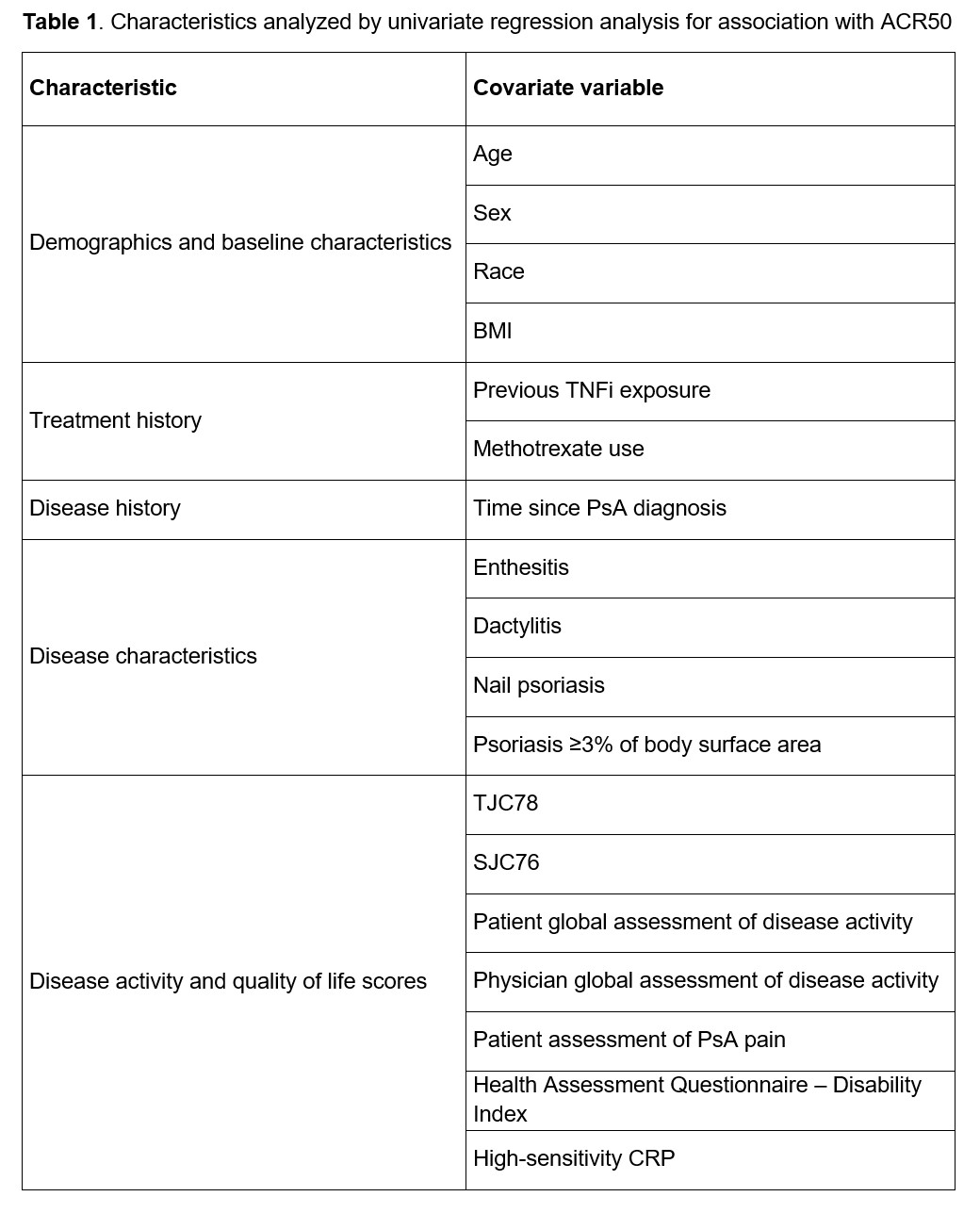
Methods: INVIGORATE-2 (NCT04209205) is a randomized, double-blind, placebo-controlled trial of IV secukinumab in patients with active PsA. Patients aged ≥18 years who fulfilled the ClASsification criteria for Psoriatic ARthritis (CASPAR) and had a diagnosis of PsA for ≥6 months were randomized 1:1 to IV secukinumab (6 mg/kg at baseline followed by 3 mg/kg every 4 weeks thereafter) or placebo (every 4 weeks) to Week 16. Only patients randomized to IV secukinumab were included in this post hoc analysis. Univariate logistic regression analyses using generalized estimating equations were performed to identify baseline characteristics (including demographics, treatment history, disease history and characteristics, and quality-of-life characteristics; Table 1) that were likely associated (P< .2) with achievement of 50% improvement in ACR response criteria (ACR50; the primary endpoint in INVIGORATE-2) from Weeks 4 to 16. These potential predictors were subsequently entered into a repeated measure multivariable logistic regression (RMMLR) model for further selection (P< .1 to keep). For the predictors kept by the RMMLR model, descriptive subgroup summaries of ACR50 response rates through Week 16 are provided. All P values derived from this post hoc analysis are nominal.
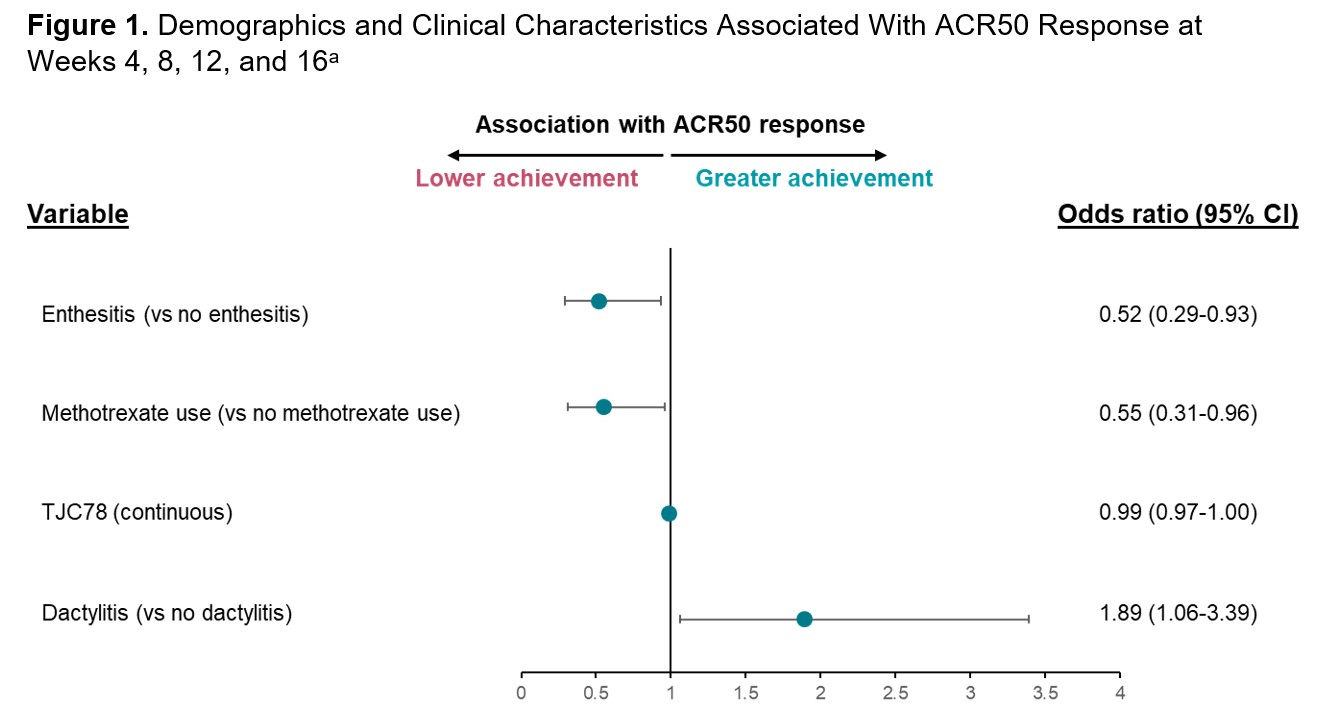
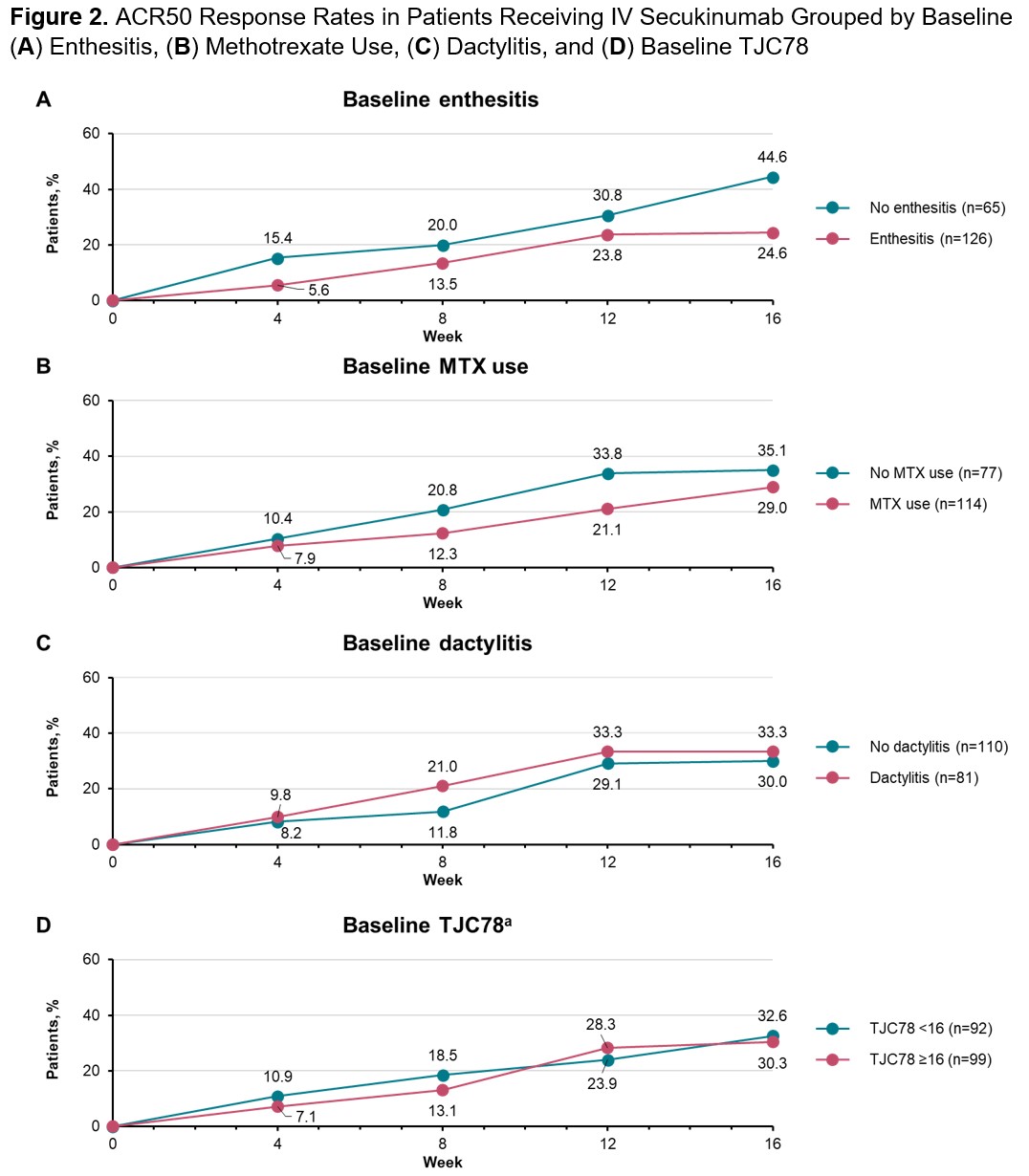
Results: A total of 191 patients with PsA who received IV secukinumab in INVIGORATE-2 were included in this post hoc analysis. The overall ACR50 response rate at Week 16 for patients receiving secukinumab in INVIGORATE-2 was 31.4%. Baseline dactylitis (odds ratio [95% CI]; 1.89 [1.06-3.39]; P=.03) was associated with greater achievement of ACR50 from Weeks 4 to 16, while baseline enthesitis (0.52 [0.29-0.93]; P=.03) and methotrexate use (0.55 [0.31-0.96]; P=.03) were associated with slightly lower achievement of ACR50 (Figure 1). Additionally, lower tender joint count in 78 joints (TJC78; continuous variable) was associated with slightly lower ACR50 response rates (0.99 [0.97-1.00]; P< .01). From Weeks 4 to 16, ACR50 response rates were higher in patients without baseline enthesitis vs those with enthesitis and in patients not taking methotrexate at baseline vs those taking methotrexate (Figure 2). In contrast, smaller differences in ACR50 response rates from Weeks 4 to 16 were seen in patients with baseline dactylitis vs those without dactylitis and in patients with TJC78 below the median value of 16 vs those with TJC78 ≥16. Other disease characteristics and demographics, including BMI, sex, and age, were not shown to be predictive of ACR50 response.
Conclusion: For patients with PsA receiving IV secukinumab in INVIGORATE-2, baseline dactylitis was associated with greater achievement of clinical response from Weeks 4 to 16, while baseline enthesitis, higher TJC78, and methotrexate use were associated with lower achievement of clinical response. However, patients experienced clinical benefit from secukinumab regardless of demographics or baseline characteristics.
a Selected from a repeated measure multivariable logistic regression model, with ACR50 at Weeks 4, 8, 12, and 16 as the response variable (P<.1).
a The median baseline TJC78 for patients included in this analysis was 16.
To cite this abstract in AMA style:
Kivitz A, Sedova L, Churchill M, Vizcaya C, Sutariya R, Cao W, Singhal A. Predictors of Clinical Response to Intravenous Secukinumab Among Patients with PsA: A Post Hoc Analysis of a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/predictors-of-clinical-response-to-intravenous-secukinumab-among-patients-with-psa-a-post-hoc-analysis-of-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-of-clinical-response-to-intravenous-secukinumab-among-patients-with-psa-a-post-hoc-analysis-of-a-phase-3-trial/