Session Information
Date: Tuesday, October 23, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster III: Treatment
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Subcutaneous secukinumab 150mg and 300mg are approved doses for the treatment of psoriatic arthritis (PsA) with the higher dose recommended for patients with anti-TNF inadequate response or moderate-to-severe psoriasis. Although multivariate logistic regression analysis can predict if a specific group of patients can benefit from the higher dose, machine learning surpasses these traditional estimation-focused analyses with a higher area under the Receiver Operating Characteristic curve. The objective is to investigate if specific baseline clinical characteristics can be used to predict which patients gain additional benefit from the 300mg dose using pooled data from the secukinumab phase 3 studies.
Methods: Bayesian Elastic net was used to analyze 4 studies (FUTURE2-5) with 2148 patients investigating a total of 275 predictors (baseline demographic, disease characteristics in both main effects and interaction effects). Eleven endpoints were analyzed at Week 16 including ACR50, PASI90, and psoriatic arthritis disease activity score (PASDAS). Heat maps were used to identify common predictors across endpoints at Week 16. PASDAS low disease activity (LDA), including remission (REM) (PASDAS LDA+REM; PASDAS score < 3.2)1, is reported as a recommended disease activity target using meta-analytic techniques. Missing responses are imputed as non-responders.
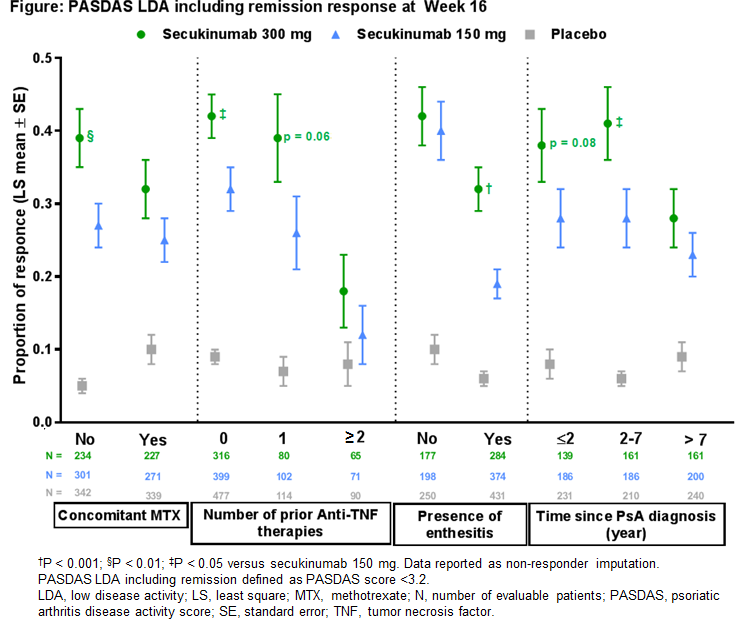
Results: There was no single predictor which alone could adequately predict which patients would achieve PASDAS LDA+REM with 300mg compared to 150mg. However, several predictors jointly produced adequate predictions: no prior use of biologic, 1 previous anti-TNF therapy, no use of concomitant methotrexate (MTX), presence of enthesitis at baseline and earlier time since PsA diagnosis (Figure).
Conclusion: Machine learning on this pooled dataset confirmed previous clinical findings for the additional benefit of secukinumab 300mg over 150mg in patients with moderate to severe psoriasis in PsA2, and in PsA patients with previous exposure to anti-TNF and in patients without concomitant MTX3. In addition, early PsA, and presence of enthesitis were identified as predictors of PASDAS LDA+REM that warrant further investigation.
References:
1. Helliwell PS and Waxman R, Ann Rheum Dis. 2018;77:467-8
2. Gottlieb et al. J Drugs Dermatol. 2015;14:821-33.
3. Kirkham et al. Ann rheum dis. 2018-eular.2374
To cite this abstract in AMA style:
Pricop L, Gaillez C, Ligozio G, Zhu X, James D. Predictors for Use of Secukinumab 300mg over 150mg in Psoriatic Arthritis: A Meta-Analysis of Four Phase 3 Trials By Machine Learning [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/predictors-for-use-of-secukinumab-300mg-over-150mg-in-psoriatic-arthritis-a-meta-analysis-of-four-phase-3-trials-by-machine-learning/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictors-for-use-of-secukinumab-300mg-over-150mg-in-psoriatic-arthritis-a-meta-analysis-of-four-phase-3-trials-by-machine-learning/