Session Information
Title: Fibromyalgia, Soft Tissue Disorders, Regional and Specific Clinical Pain Syndromes: Research Focus
Session Type: Abstract Submissions (ACR)
Background/Purpose: The number of symptoms and comorbidities associated with the chronic pain condition of fibromyalgia (FM) complicates its identification and diagnosis.
Methods: This retrospective analysis used structured de-identified electronic health records from the 2011-2012 Humedica database, including demographics, clinical characteristics, and healthcare resource use. An FM cohort was defined as subjects ≥18 years in 2011 with ≥2 ICD-9 codes for FM (729.1) ≥30 days apart during 2012; the no-FM cohort did not have the ICD-9 code. Univariate analyses characterized between-cohort differences and determined the demographic, clinical, and healthcare resource variables associated with an FM diagnosis. A Random Forest (RF) model was used to predict FM and non-FM subjects by entering all variables into the model (1500 bootstraps) with internal down-sampling (to account for imbalance between cohorts). Importance of the variables was computed using RF to determine the trade-off on accuracy when only the top 10 variables were fitted. For practical clinical application, a rule-based model was used to derive simple statements to help explain which patient sets have the largest effect on the likelihood of an FM diagnosis.
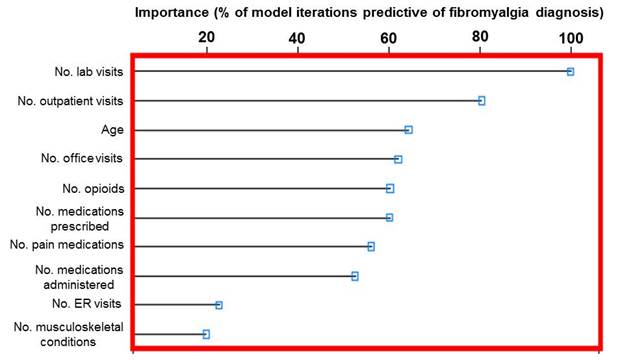
Results: Significant differences were observed between the FM (n=4,296) and no-FM (n=583,665) cohorts for demographics (P<.0001) except for age, for most evaluated comorbidities (P<.0001), and for healthcare resource use (P<.0001), with more comorbidities and resource use in FM subjects. Resources included proportions of subjects with utilization, and units used per subject for emergency room visits, outpatient visits, hospitalizations, and medications. The top 10 variables for predicting an FM diagnosis were identified from the RF models based on level of importance as reflected by the percent of model iterations in which the variable was predictive (Figure). A receiver operator characteristic curve confirmed the predictive accuracy of the model variables (area under the curve of 0.810). Rules were developed to identify patients with high predicted probability of an FM diagnosis; e.g., the average predicted probability of 0.54 for a subset of patients with outpatient visits excluding office visits >0 and prescriptions administered/ordered ≤3 and the number of musculoskeletal pain conditions >0 was more than double the 0.22 predicted probability for those not in the subset. Similarly, a rule to identify non-FM (opioid prescriptions administered/ordered/written =0; visits where diagnostic/laboratory tests were ordered =0; and number of musculoskeletal pain conditions =0) correctly classified 100% of the sample.
Conclusion: Random forest modeling can be applied to determine likelihood of an FM diagnosis. Rules can simplify this method with good accuracy. Further validation of RF may help facilitate earlier diagnosis and enhance management strategies.
Disclosure:
B. Emir,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
J. Mardekian,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
E. T. Masters,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
A. Clair,
Pfizer Inc.,
1,
Pfizer Inc.,
3;
M. Kuhn,
Pfizer Inc.,
3;
S. L. Silverman,
Amgen, Eli Lilly, Pfizer,
8,
Amgen, Genentech, Eli Lilly, Novartis, Pfizer,
5,
Amgen, Eli Lilly, Medtronics, Pfizer,
2.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictive-modeling-of-a-fibromyalgia-diagnosis-increasing-the-accuracy-using-real-world-data/