Session Information
Date: Wednesday, November 13, 2019
Title: 6W023: Systemic Sclerosis & Related Disorder – Clinical III: Predictors of Outcome (2912–2917)
Session Type: ACR Abstract Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Autologous hematopoietic stemcell transplantation (HSCT) has shown to improve survival of SSc patients with poor prognosis, but is hampered by treatment related mortality (TRM). Better selection of eligible patients could improve outcomes.
Objectives: 1. To evaluate event-free survival, TRM and response after HSCT in SSc and 2. To explore patient characteristics that associate with events.
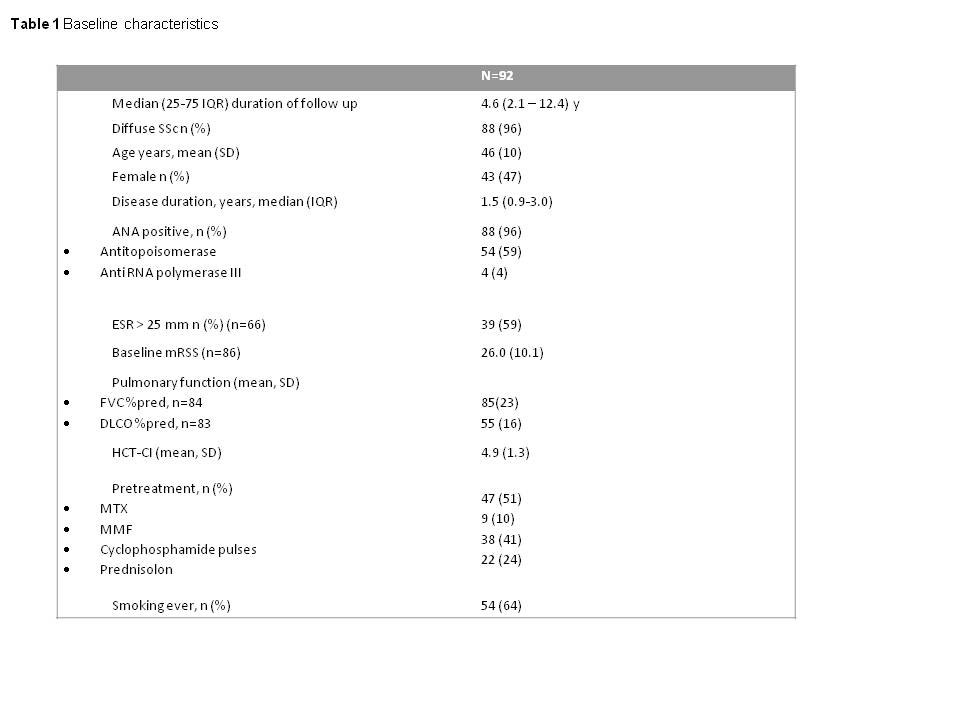
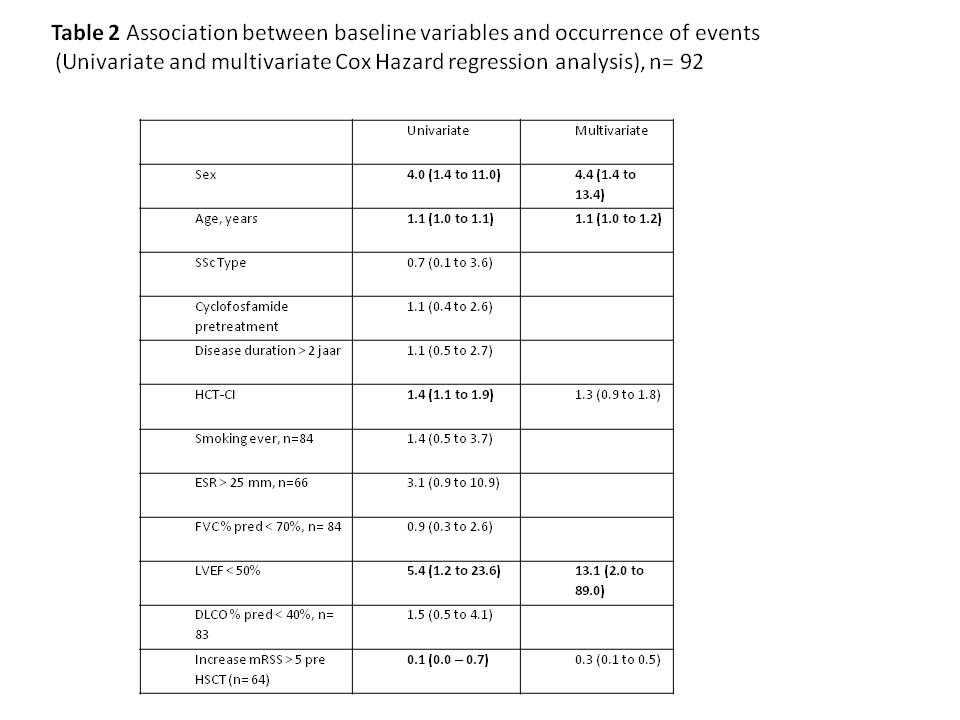
Methods: Data on event-free survival of all patients treated with HSCT for SSc in the Netherlands between 1998 and 2017, performed as previously described (1) and with ≥ 1 year of follow-up were collected. Baseline characteristics including hematopoietic cell transplant comorbidity index (HCT-CI) (2) and left ventricular ejection fraction (LVEF) and data for skin involvement (modified Rodnan skin score (mRSS)), pulmonary function (forced vital capacity % predicted (FVC% pred), diffusion capacity % predicted (DLCO% pred)) and staging for interstitial lung disease on high resolution CT scan (HRCT) assessed according Goh (3) were collected at baseline and at 1,2, 5 years ; data for events and death were collected until end of study. All deaths were discussed in a consensus meeting (SB, MB, JV, MV) and classified as TRM, SSc progression or other. Event-free survival was defined as described before (4). Relapse was defined as an increase in mRSS of > 25% and > 5 points compared to the lowest value since HSCT, or either a ≥10% decline in FVC% pred, or ≥5 – < 10% decline in FVC% pred and ≥15% decline in DLCO% pred or initiation of immunosuppression (4). The association between event-free survival and baseline characteristics was examined by univariate Cox regression analysis. Factors with a significant association were entered in a multivariate analysis on imputed missing data.
Results: In total 92 patients were included (Table 1). Event-free survival estimates at 5, 10 and 15 years were 0.79, 0.79 and 0.68 respectively. Twenty deaths occurred, which were classified as TRM (n=10, 11%), SSc progression (n=4, 4%) and other (n=6 7%). Relapse occurred in 22 patients in the first 5 years: skin relapse in 5, lung relapse in 11 and initiation of immunosuppression in 12 (in 7 patients without skin or lung relapse). FVC, DLCO, mRSS and Goh scores improved significantly over time in patients surviving the first 5 years (n=75) (Figure 1). Events were independently associated with male sex, LVEF < 50% and older age (table 2).
Conclusion: Event-free survival at 10 years after HSCT for SSc was 79%; male sex, lower LVEF and older age were identified as independent risk factors for events. Our data confirms efficacy of HSCT in improving survival and skin and lung involvement.
Refs:1. van Laar JM etal. JAMA. 2014;311(24):2490-8. 2. Sorror ML etal. Biol Blood Marrow Transplant. 2015;21(8):1479-87.3. Goh NS etal. Am J Respir Crit Care Med. 2008;177(11):1248-54.4. Khanna D etal.The Journal of rheumatology. 2015;42(11):2168-71.
A skin involvement as measured by mRSS; B FVCpred: forced vital capacity % predicted; C DLCO pred: diffusion capacity % predicted; D Total Goh scores
To cite this abstract in AMA style:
van Bijnen S, Boonstra M, van den Ende E, Kroft L, Geurts B, Snoeren M, Schouffoer A, Spierings J, van Laar J, Huizinga T, Voskuyl A, van der Velden W, van den Hoogen F, de Vries-Bouwstra J, Vonk M. Predictive Factors for Treatment Related Mortality and Event-Free Survival After Autologous Hematopoietic Stem Cell Transplantation for Systemic Sclerosis: Results of a Long Term Follow-up Multi-centre Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/predictive-factors-for-treatment-related-mortality-and-event-free-survival-after-autologous-hematopoietic-stem-cell-transplantation-for-systemic-sclerosis-results-of-a-long-term-follow-up-multi-centr/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictive-factors-for-treatment-related-mortality-and-event-free-survival-after-autologous-hematopoietic-stem-cell-transplantation-for-systemic-sclerosis-results-of-a-long-term-follow-up-multi-centr/