Session Information
Date: Sunday, November 8, 2015
Title: Rheumatoid Arthritis - Small Molecules, Biologics and Gene Therapy Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: : Currently,
there is no clarity regarding which biologic to switch to when patients fail to
respond to TNF inhibitors (TNFis). Detailed information for
predicting long-term outcomes when switching from TNFis
to ABT would be helpful in clinical practice. This study aimed to identify predictive
factors for achieving low disease activity (LDA) in rheumatoid arthritis (RA)
patients switching from tumor necrosis factor inhibitors to abatacept
(ABT).
Methods: Patients who were registered in the
multicenter observational cohort study in Japan were enrolled in this study.
Predictive factors for LDA achievement at each time point were determined by
univariate and multivariate logistic regression analyses. The cut-off of
DAS28-CRP and
LDA achievement at 52 weeks were explored using receiver operating
characteristic (ROC) curves.
Results: Of
2,771 RA patients registered until 2013, 76 with moderate or high disease
activity were selected. Fifty-three
percent of patients received ABT with MTX (mean dose; 7.8 mg/week), and 26%
achieved LDA. In total, the drug
retention rate at 52 weeks was 76.3%.
Multivariate
analysis confirmed that
DAS28-CRP at 12 weeks and
at 52 weeks [OR: 0.26, 95% confident interval (CI): (0.12-0.56), OR: 0.25, 95%
CI: (0.11-0.57), respectively]. DAS28-CRP at baseline was not the factor.
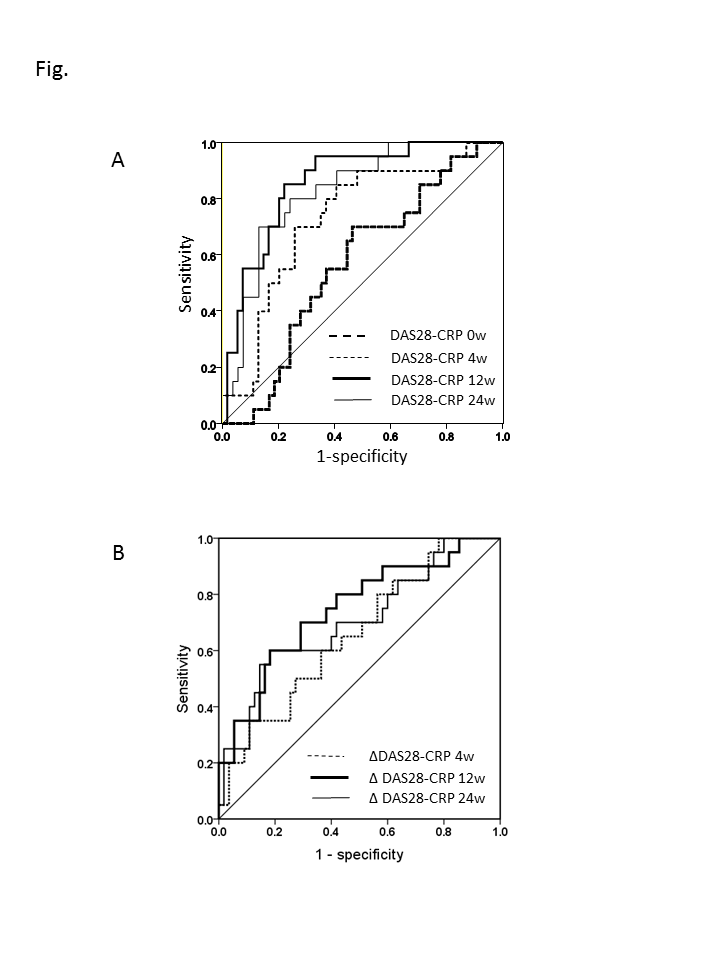
We confirmed the association between DAS28-CRP at each
time point and LDA achievement at 52 weeks based on ROC curves. DAS28-CRP at 4,
12, and 24 weeks was associated with LDA achievement (AUC at 4 weeks, 0.74; 12
weeks, 0.86; 24 weeks, 0.83) (Fig. A), and that between DDAS28-CRP from
baseline to each time point (AUC from baseline to 4 weeks, 0.66; to 12
weeks, 0.75; to 24 weeks, 0.70) (Fig. B).
The best cut-off values of DAS28-CRP at 12 weeks and
patients who achieved both of these cut-off values at 12 weeks achieved LDA at
52 weeks.
Conclusion: We examined the effectiveness of switching from TNFis to ABT in clinical practice. Our findings suggest the
need to consider the clinical course up to 12 weeks in order to predict
long-term outcomes. These findings should be useful and applicable to the
“treat to target” strategy in real-world clinical settings.
To cite this abstract in AMA style:
Kojima T, Takahashi N, Funahashi K, Asai S, Takemoto T, Asai N, Watanabe T, Ishiguro N. Predictive Factors for Achieving Low Disease Activity at 52 Weeks after Switching from Tumor Necrosis Factor Inhibitors to Abatacept: Results from a Multicenter Observational Cohort Study of Japanese Patients [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/predictive-factors-for-achieving-low-disease-activity-at-52-weeks-after-switching-from-tumor-necrosis-factor-inhibitors-to-abatacept-results-from-a-multicenter-observational-cohort-study-of-japanese/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/predictive-factors-for-achieving-low-disease-activity-at-52-weeks-after-switching-from-tumor-necrosis-factor-inhibitors-to-abatacept-results-from-a-multicenter-observational-cohort-study-of-japanese/