Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
As treatment paradigms for rheumatoid arthritis (RA) continue to evolve, population-based studies can help assess which strategies are being used in “real-world” practice for the complete spectrum of clinical RA. This approach can also relate therapy to the consumption of other health-care resources such as RA-related surgery or hospitalization which are fundamental for RA economic models. Herein we describe treatment patterns in RA patients newly diagnosed in 2009 and followed for two years.
Methods:
A retrospective cohort analysis of adults (age ≥20) with complete medical and pharmacy insurance claims from 1 Jan 2008 to 31 Dec 2011 was used. “Incident RA” required a primary diagnosis at two separate visits 30 days apart in 2009 and no prior claims for RA. Databases employed were the Marketscan Commercial Claims and Encounters (CCAE) and Medicare Supplemental and Coordination of Benefits (MSCB) databases from Truven Health Analytics. Information on demographics, co-morbidities and treatments including conventional (cDMARD) and biologic (bDMARD) therapy and sequencing thereof was obtained for the entire follow-up period. Selected results were informally compared to a similar analysis in 2006 [Arth Rheum 2010;62(Suppl10):18].
Results:
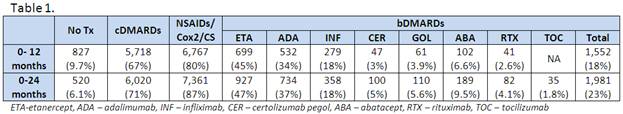
There were 8.02 million persons who met study criteria from which 8,507 RA cases were newly diagnosed in 2009. Incidence was 1.06/1000 persons at risk (1. 5 in females; 0.57 in males). 18% of incident patients received a bDMARD in year one (versus 21% in 2006) and 67% a cDMARD. Among the former, TNF inhibitors were the most commonly prescribed (94%). Over two years of follow-up, 23% of patients were prescribed bDMARDs but 22% of them were subsequently switched to a different biologic. A total of 26% of patients did not receive a DMARD over the two years (as compared to 28% in 2006). Corticosteroids were given to 77% of patients during the 2-year follow-up.
Treatment patterns over the 24 months after RA diagnosis were as follows (Table 1):
Of patients who used bDMARDs, 78% had only one, 17% had two, 4% had three, and 1% had four different biologics over the study period. The most common first line biologic was ETA (45%), followed by ADA (27%), and INF (22%), while second line biologic choices were ADA (43%), INF (22%), and ABA (13%).
Conclusion:
The majority of newly diagnosed patients received anti-rheumatic therapy. The proportion of cDMARDs and bDMARDs were similar in 2006 and 2009. Switching among biologics was not uncommon. There were 26% (versus 28% in 2006) who had not received anti-rheumatic treatment over the two years of follow-up.
Disclosure:
M. M. Crane,
GlaxoSmithKline,
3,
GlaxoSmithKline,
1;
B. Stoykova,
GlaxoSmithKline,
1,
GlaxoSmithKline,
3;
J. Priest,
GlaxoSmithKline,
1,
GlaxoSmithKline,
3;
N. Wang,
GlaxoSmithKline,
3;
H. Krzywy,
GlaxoSmithKline,
1,
GlaxoSmithKline,
3;
R. Ganguly,
GlaxoSmithKline,
1,
GlaxoSmithKline,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/population-based-analysis-of-treatment-patterns-for-recently-diagnosed-rheumatoid-arthritis-patients-in-the-united-states/